Abstract
Background: Acute myeloid leukemia (AML) is an aggressive hematologic malignancy with a poor prognosis in the adult population, despite recent advancements in treatment paradigm. Induction with the liposomal formulation of cytarabine and daunorubicin (CPX-351) which has been shown in phase III trials to improve the median overall survival (OS) compared to standard induction (9.6 versus 5.9 months, respectively) and is FDA approved for patients therapy related AML or AML with myelodysplasia-related changes. Hypomethylating agents (HMA) (azacitidine and decitabine) have recently garnered renewed attention in combination with the BCL-2 inhibitor venetoclax (VEN). Azacitidine with venetoclax demonstrated a median overall survival of 16.9 months in those that were deemed ineligible for intensive induction. While these trials were inherently different patient populations, in clinical practice, physicians must often choose between these two therapies in the frontline setting. Thus, we aimed to examine patient characteristics and respective outcomes with these two treatment approaches.
Methods: This was a retrospective cohort studies of patients diagnosed with AML and treated at Virginia Commonwealth University/Massey Cancer Center as identified by our data analytics core between June of 2018 and December of 2020. Data was extracted and manually verified from the electronic medical record into an AML database instrument created in RedCap. Statistical analysis was then performed using GraphPad Prism. Descriptive analyses were conducted for demographics and baseline clinical characteristics, and OS rates were evaluated using Kaplan-Meier analyses and compared using log-rank test.
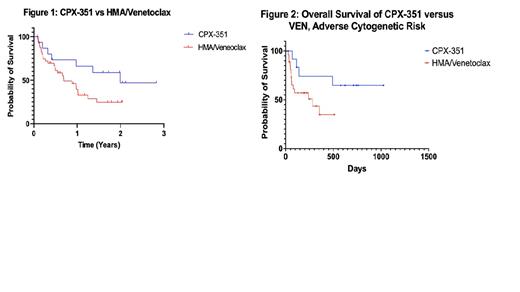
Results: A total of 160 patients with AML were identified. Of those, 58 were treated with either HMA/VEN (N = 43) or CPX-351 (N = 15) in the frontline setting. Median age of patients treated with HMA/VEN was 72 (range: 37 - 85) compared to the median age of 69 (range: 44 - 72) for the CPX-351 group, which was statistically significant (p = 0.002). There was no statistical difference in other demographic characteristics such as sex (p = 0.380), race (p = 0.323), Charleston Comorbidity Index (p = 0.097), or ECOG score (p = 0.126). AML risk stratification based on molecular and cytogenetic data per ELN 2017 also did not differ between the two groups (p = 0.230), with both groups having numerically similar adverse-risk patients (86.7% in the CPX-351 cohort and 70.7% in the VEN cohort). Figure 1 demonstrates a Kaplan-Meier analysis, which revealed a median OS in the VEN group of 281 days (9.23 months) compared to a median OS in the CPX-351 group of 713 days (23.4 months), which was not significant (p = 0.109). Additionally, there was no difference in the OS when only the adverse cytogenetic categories were analyzed from each cohort (Figure 2, p = 0.058). There was one (6.7%) death within 30 days in the CPX-351 group and no deaths within 60 days, compared to one (2.3%) death within 30 days and 9 (20.9%) deaths within 60 days in the VEN group, which was not significantly different (p = 0.257). Allogeneic stem cell transplant was more common in the CPX-351 group (40%) than the VEN group (4.7%) (p = 0.0007).
Conclusion: Although these regimens were intended for diverging populations of induction versus non-induction candidates, there is clinical overlap in physician discretion regarding both regimens. Our data shows similar baseline characteristics between these two groups, though the VEN cohort was slightly older. Our data demonstrated that in the CPX-351 cohort, the OS was longer, there appeared to be fewer deaths during induction, and more patients proceeded to transplant - though the former approached statistical significance, particularly when matched with adverse cytogenetic risk (p = 0.058). This argues for continued use of intensive regimen for those who are candidates, especially those in whom allogeneic hematopoietic stem cell transplant is being considered. Prospective studies between these regimens is warranted.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal