Abstract
Introduction:
MGMT is a key enzyme in DNA repair. Epigenetic silencing of the MGMT gene plays a critical role in carcinogenesis and confers increased sensitivity of tumor cells to alkylators. MGMT methylation status is known to be prognostic and predictive of treatment response to Temozolomide in patients with Glioblastoma Multiforme (GBM). Despite advancing knowledge about the clinical implication of MGMT in GBM, there is limited information about the association between tumor MGMT status and hematologic tolerance to Temozolomide. The objective of this study was to evaluate the association of MGMT status and hematologic toxicities after treatment with Temozolomide.
Methods:
A single institutional retrospective case control study was performed for patients with biopsy proven diagnosis of GBM within the years 2015 to 2020. We collected tumor specific information such as patients MGMT status and treatment specific information including the timing of hematologic adverse events, dose exposure, incidence, and severity of hematologic toxicities. To evaluate the association between tumor MGMT status and hematologic toxicities to Temozolomide we used the Fisher's exact test as well as odds ratio with 95% confidence interval.
Results:
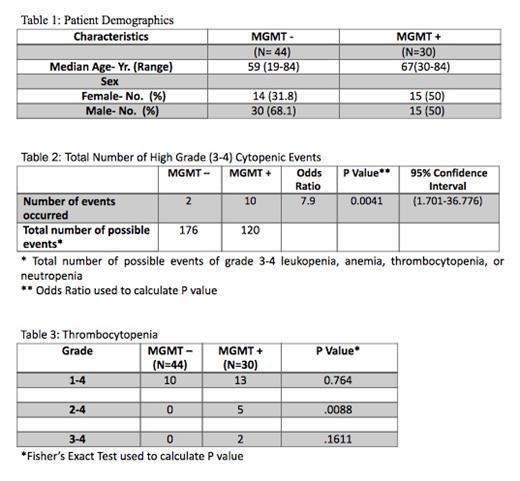
A total of 74 patients with biopsy proven glioblastoma were evaluated. 44 were MGMT negative while 30 were MGMT positive. All hematologic adverse events occurred within six weeks of exposure to Temozolomide. At the time of the study completion, the number of high grade (grade 3-4) hematologic toxicity events including leukopenia, anemia, thrombocytopenia, or neutropenia were evaluated. There were 2 out of a possible 176 events for nonmethylated MGMT vs 10 out of a possible 120 events for methylated MGMT. This association was determined to be statistically significant with a P value of 0.0051 by Fisher's exact test. Odds ratio (OR) was also calculated with a OR of 7.9, P value of 0.0041 and 95% confidence interval of [1.701- 36.776]. Statistical difference was also noted in the incidence of grade 2-4 thrombocytopenia with 5 events occurring in the methylated group and 0 events occurring in the nonmethylated group with a P value of 0.0088 by Fisher's exact test.
Conclusion:
Studies have shown the importance of MGMT status in predicting response to treatment with alkylators in patients with GBM. Our study shows that the patients with MGMT methylated GBM tend to develop more hematologic toxicities compared to patients with MGMT non-methylated GBM early in their treatment course, leading to early discontinuation of Temozolomide. This implies these patients need to have close monitoring for hematological toxicity from the initiation of treatment. Further research is warranted to investigate the effect of tumor MGMT status on the DNA repair network in host hematopoietic system after exposure to alkylators.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal