Abstract
Natural killer cells (NK) are components of the innate immune system which play an important role in surveillance of cancer cells. Despite promising results in clinical trials, the use of NK-based therapies is limited due to safety issues. Exosomes are nanosized extracellular vesicles secreted by all types of cells. In recent years, exosomes have emerged as a powerful, natural therapeutic tool due to their low immunogenicity, low cytotoxicity, prolonged bioavailability and superior targeting ability. Since exosomes are known to carry cargo that reflects their cell of origin, we were prompted to test whether NK-derived exosomes (NK exo) maintain the anti-leukemia capacity of NK cells. To test the feasibility of this approach we undertook a set of preliminary experiments and evaluated the anti-leukemic potential of NK exo in-vitro on a wide variety of leukemic cell lines and patient-derived samples while also striving to assess their killing potential in-vivo using a murine xenograft model . This study aims to provide the pre-clinical evidence needed to test the NKexo approach in future clinical studies with the intention of ultimately developing an acellular "off-the-shelf" product to treat leukemia.
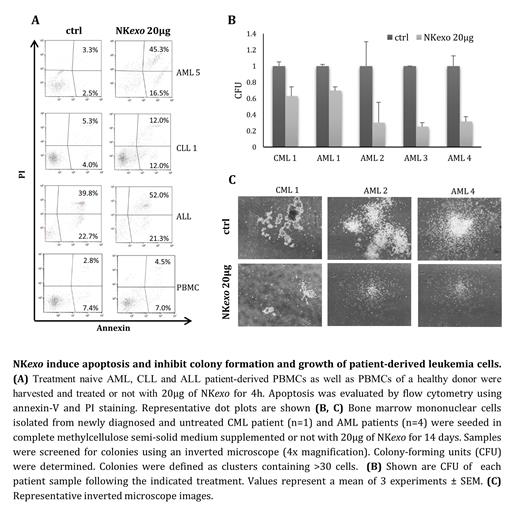
Initially, we isolated NK exo from the engineered NK92MI cell line by ultracentrifugation and demonstrated the presence of large amounts of 100-200nm cap-shaped particles by means of nano-tracking-analysis and transmission-electron-microscopy. We then showed that these particles express the exosomal-characteristic CD63- and CD81- and NK-characteristic CD56-biomarkers. Next, we evaluated the effect of NK exo on CML (K562), T-ALL (Jurkat), B-ALL (UoC) and AML (HL-60 and KG1a) cell lines. NK exo treatment initiated a time- and dose-dependent reduction in viability and induction of apoptosis in all cell lines tested. The effect of NK exo exposure on cell viability and apoptosis was reduced in the presence of the pan-caspase inhibitor Z-VAD-FMK suggesting that NK exo exert their cytotoxic effect in a caspase-dependent manner. In addition, exposure to NK exo led to a significant increase in apoptosis in treatment naive AML, CLL and ALL patient-derived mononuclear cells. Moreover, we witnessed a significant reduction in colony formation of mononuclear cells harvested from treatment naive CML and AML patients in the presence of NK exo. While leukemia cells were targeted and severely affected by NK exo, healthy B-cells remained unaffected, indicating a selective effect. The selective trait of NK exo was also confirmed via an exosome uptake assay which demonstrated that NK exo were taken up specifically by leukemic cells but not by healthy B-cells.
In an in-vivo xenograft model for AML; NOD-SCID IL2Rgama null (NSG) mice were engrafted with the well characterized human AML cell line HL60, and were then intravenously injected with NK exo. Our preliminary results indicate that NK exo treatment improves overall mice survival indicating an anti-leukemic effect of NK exo in-vivo.
In this "proof-of-concept" study, we show that NK exo, similar to their cell-of-origin, have a strong and selective anti-leukemia effect. To provide the pre-clinical evidence needed to test the NK exo approach in future clinical studies, we are currently continuing to test the anti-leukemic potential of NK exo in-vivo on a xenograft murine model. Our preliminary results propose the use of NK exo as a novel therapeutic platform to combat leukemia. More broadly, since NK cells target a wide range of cancer cells, not just leukemia cells, and since we have shown that NK exo maintain the anti-leukemic capacity of their donor cells, this approach may become applicable in other hematological and solid malignancies.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal