Abstract
Background:
Natural killer cells (NK) are known to be the first cells that recover after allogeneic hematopoietic stem cell transplant (HCT). NK cells have anti-leukemic and anti-viral properties and are also implicated in the graft versus leukemia (GVL) effect without causing graft versus host disease (GVHD). There is emerging evidence supporting the use of NK cells post-transplant as adoptive immunotherapy to prevent or treat the relapse of hematologic malignancies. Here, we present a systematic review and meta-analysis aimed to investigate the outcomes with NK cells infusion after HCT.
Methods:
A detailed literature search was conducted for the systematic review and meta-analysis according to the guidelines mentioned in the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA). Population, intervention, comparison, and outcome (PICO) table was developed and three electronic databases (PubMed, Cochrane Library, and ClinicalTrials.gov) were searched through January 2021 using MeSH terms and keywords for "hematologic malignancy and natural killer cells" and "hematopoietic stem cell transplantation and natural killer cells". No filters or publication time limits were applied for the search. A total of 988 records were identified through database searching. After excluding duplicates, review, and non-relevant articles, we selected 9 studies that reported outcomes with NK cells infusion after HCT. Quality evaluation was done using the NIH quality assessment tool and Inter-study variance was calculated using the Der Simonian-Laird Estimator. Pooled analysis was done using the 'meta' package (Schwarzer et al, R programming language) and proportions with 95% confidence intervals (CI) were computed.
Results:
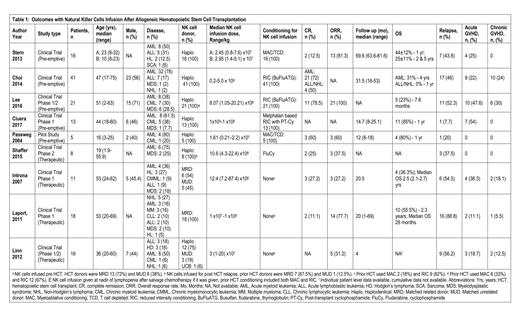
We identified 9 studies with a total of 149 participants. Out of these, 53 patients were from 4 studies using NK cells for the treatment of relapsed disease after HCT (therapeutic studies) and 96 patients were from 5 studies using NK cells to prevent relapse after HCT (pre-emptive studies).
Therapeutic use of NK cells for relapsed disease after HCT: A total of 53 patients from 4 studies were evaluated. The median age of patients was 44.5 (1.9-69) years, and 40% patients were male. The median follow-up was 20 (1-69) months. Overall survival (OS) was reported by two studies at 2 years and was 36.3% and 55%. The pooled complete response (CR) was 18% (95% CI 0.06-0.33, I 2 =0%, n=37) and overall response rate (ORR) was 45% (95% CI 0.20-0.71, I 2 =71%, n=53). The pooled relapse rate was 65% (95% CI 0.32-0.92, I 2 =75%, n=42). The pooled incidence of acute GVHD and chronic GVHD was 15% (95% CI 0.03-0.31, I 2 =41%, n=53) and 8% (95% CI 0.01-0.18, I 2 =0%, n=53) respectively.
Pre-emptive use of NK cells to prevent relapse after HCT: A total of 96 patients from 5 studies were evaluated. The median age of patients was 33.5 (2-75) years, and 48% patients were males. The median follow-up was 23.1 (8-81.6) months. The median OS was 1 (0.63-5) years. The pooled CR was 45% (95% CI 0.21-0.70, I 2 =76%, n=83) and ORR was 89% (95% CI 0.57-1.00, I 2 =77%, n=42). The pooled relapse rate was 35% (95% CI 0.20-0.52, I 2 =55%, n=96). The pooled incidence of acute GVHD and chronic GVHD was 30% (95%CI 0.14-0.48, I 2 =61%, n=96) and 8% (95%CI 0.00-0.24, I 2 =73%, n=96) respectively.
Conclusion:
Infusion of NK cells after HCT to prevent relapsed disease results in favorable outcomes with an acceptable toxicity profile. Optimal use of NK cells infusions after HCT is in a pre-emptive fashion to prevent relapsed disease and the efficacy of NK cells infusions after HCT in the setting of overt hematologic relapse is modest. Our findings suggest the need for large prospective clinical trials to establish the potential benefit of NK cells infusion after HCT to prevent relapse of hematologic malignancies without increased non-relapse mortality.
Abhyankar: Incyte/Therakos: Consultancy, Research Funding, Speakers Bureau. McGuirk: Bellicum Pharmaceuticals: Research Funding; Gamida Cell: Research Funding; Novartis: Research Funding; Novartis: Research Funding; EcoR1 Capital: Consultancy; Fresenius Biotech: Research Funding; Astelllas Pharma: Research Funding; Magenta Therapeutics: Consultancy, Honoraria, Research Funding; Juno Therapeutics: Consultancy, Honoraria, Research Funding; Allovir: Consultancy, Honoraria, Research Funding; Pluristem Therapeutics: Research Funding; Kite/ Gilead: Consultancy, Honoraria, Other: travel accommodations, expense, Kite a Gilead company, Research Funding, Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal