Abstract
Introduction: Platelet function disorders (PFDs) are a group of heterogenous bleeding disorders with varying bleeding phenotype. Intraoperative and post-operative bleeding are serious complications among patients with PFDs undergoing surgery. There are very few studies in literature that have specifically investigated surgery associated bleeding complications in PFDs. The aim of this study was to utilize a large national dataset to describe surgeries performed in patients with PFD, characterize the bleeding associated with these surgical procedures and outline the therapeutic approaches adopted.
Methods: In this retrospective study, the ATHNdataset was queried for demographic data, PFD diagnosis, surgeries among patients with PFD, intraoperative and post-operative bleeding episodes and treatment. Descriptive statistics were used. The ATHNdataset captures information from patients with bleeding and clotting disorders from over 140 federally funded hemophilia and thrombosis treatment centers (HTCs) in the US. Patients authorize inclusion of their demographic and clinical information in this de-identified Health Insurance Portability and Accountability Act (HIPAA)-compliant data set.
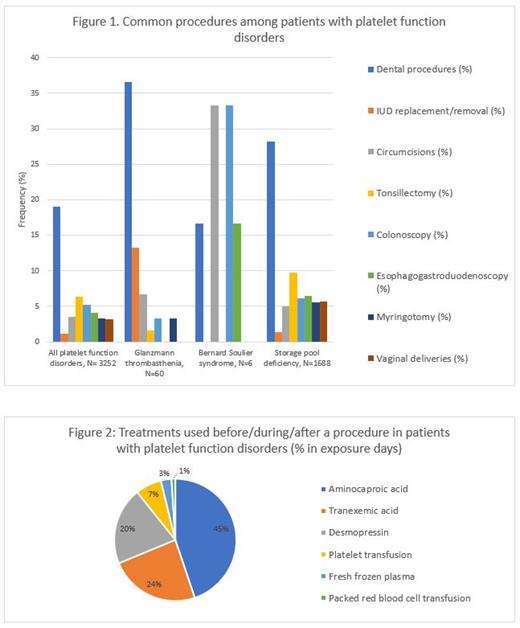
Results: From January 2010 to March 2020, the ATHNdataset captured 2767 patients with PFDs, of which 1769 (63.93%) were female and 998 (36.1%) were male, with 1393 patients between 0-18 years (50%) and 1374 (50%) adults >18 years. PFDs identified include 32 patients with Bernard Soulier syndrome (1.16%), 131 patients with Glanzmann thrombasthenia (4.7%), 4 patients with Gray platelet syndrome (0.14%), 29 patients with Hermansky Pudlak syndrome (1%), 1548 patients with storage pool deficiency (55.9%), and 1023 patients diagnosed as PFD (36.9%). A total of 3252 procedures were reported between 2010 and 2020; 1271 patients (46%) patients with at least one documented procedure. Figure 1 shows common procedures among patients with PFDs.
Surgery-associated bleeding episodes (includes intraoperative and post-operative bleeds) were reported with 69 procedures (2.1%), which included intraoperative bleeds reported for 18 procedures (0.5%) and post-operative bleeds reported for 51 procedures (1.6%). Of the 60 procedures in patients with Glanzmann thrombasthenia, surgery-associated bleeding episodes were reported after 9 dental procedures (41%), 1 circumcision (25%) and 11 other surgeries/procedures (18.3%). Of the 6 procedures in patients with Bernard Soulier syndrome, no intraoperative or post-operative bleeding episodes were reported. Of 1688 procedures in patients with storage pool deficiency, surgery-associated bleeding episodes were reported after 26 dental procedures (1.5%) and 62 other surgeries/procedures (3.67%). No intraoperative or post-operative mortality was reported among these patients.
Of 1272 patients who underwent at least 1 procedure, 646 patients (50.7%) received some form of treatment before/during/after a procedure. Among these 646 patients, 2794 exposure days of hemostasis medications were used before/during/after procedures. Among these, 49% were prior to the procedure, 0.7 % during the procedure and 49.5% after the procedure. Treatments used are shown in figure 2.
Conclusion: Our study shows that patients with PFDs have a substantial risk of bleeding associated with surgery. Identifying the risk of bleeding by type and providing appropriate pre-surgical prophylaxis can decrease rates of surgery-associated bleeding in PFDs.
Sidonio: Sanofi, Takeda, Octapharma, Bayer, Biomain, Grifols, Kedrion, Genentech. Catalyst, Guardian Therapeutics, Novo Nordisk, Hema Biologics, Uniqure.: Consultancy, Honoraria. Ahuja: Genentech: Membership on an entity's Board of Directors or advisory committees; Takeda: Other: DSMB member ; XaTek, Inc: Patents & Royalties; Sanofi: Membership on an entity's Board of Directors or advisory committees.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal