Abstract
Background: Pediatric myelodysplastic syndromes (MDSs) are a heterogeneous group of clonal disorders, accounting for less than 5% of childhood hematologic malignancies. Usual indications to HSCT are: MDSs with excess of blasts, MDSs secondary to previously administered chemoradiotherapy and RCC associated with monosomy 7, complex karyotype, severe neutropenia, or erythrocyte/platelet transfusion dependence [Locatelli & Strahm, Blood 2018]. We previously demonstrated that TBdepl-haploHSCT is a suitable option for children with acute leukemia, with outcomes comparable to those reported in studies using either an HLA-identical sibling or an unrelated volunteer as donor. Here we present the results of this approach in children with MDSs.
Patients and methods: Between February 2013 and February 2021, 23 children with MDSs other than juvenile myelomonocytic leukemia received TBdepl-haploHSCT from an HLA-partially matched relative at Ospedale Pediatrico Bambino Gesù, Rome, Italy or at IRCCS Fondazione Policlinico San Matteo, Pavia, Italy as part of a prospective study (#NCT01810120). All patients were prepared to the allograft using a fully-myeloablative conditioning regimen including a combination of cytotoxic drugs and/or total body irradiation (TBI). Anti-T-lymphocyte globulin (ATLG) was used before transplantation (12 mg/kg total dose, from days -5 to day -3) to modulate bi-directional donor/recipient alloreactivity. Rituximab (200 mg/sqm) was administered on day -1 to prevent post-transplantation EBV-induced lymphoproliferative disorders (PTLD). No patient received any post-transplant pharmacological GvHD prophylaxis.
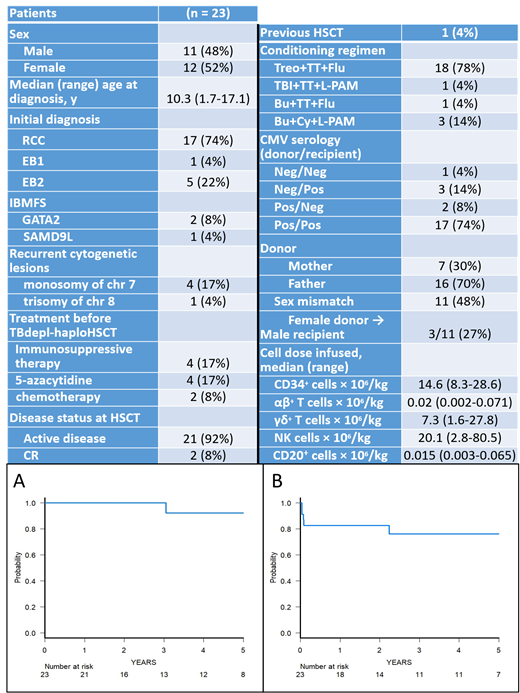
Results: Characteristics of patients enrolled in the study are shown in Table 1 (which reports also donor and graft characteristics). Median follow-up of surviving patients is 4.2 years (range: 0.5 - 8.5 years). Seventeen children were affected by refractory cytopenia of childhood (RCC) (2 cases occurring in the context of inherited bone marrow failure syndromes: one had GATA2 deficiency and the other SAMD9L mutation), while 1 and 5 were affected by MDS with excess of blasts 1 (EB1) and EB2 (one had GATA2 deficiency), respectively. Median time to neutrophil and platelet recovery was 14 (range 10-19) and 11 (range 9-14) days, respectively, with four patients (3 with RCC and 1 with EB2) experiencing primary graft failure, the cumulative incidence of this complication being 17.3% (95% CI 0.3-31.5). All these 4 patients were rescued with a second TBdepl-haploHSCT from the same or the other parent. Cumulative incidence of grade II-III acute GvHD was 11.4% (95% CI 0-25.2). One patient developed skin and gut GvHD after the second TBdepl-haploHSCT, while for all other patients skin was the sole organ involved; no case of grade IV GvHD was observed. One patient developed moderate chronic GvHD [cumulative incidence 5.2% (95% CI 0-14.8)], which completely resolved with low-dose steroids and ruxolitinib. Notably, no patient died for transplant-related complications. Six patients experienced CMV, 2 HHV-6 and 1 adenoviral infection/reactivation; one patient developed lung aspergillosis, which resolved with specific treatment. One patient affected by EB2, not in remission at time of transplant, relapsed 27 months after HSCT, the 5-year cumulative incidence of relapse being 7.1% (95% CI, 0-19.7); she eventually died after failing a second HSCT. The 5-year probability of overall and event-free survival were 92.3% (95% CI 56.6 -98.9) and 76.3% (95% CI 51.3-89.6) (Figure 1A and B), respectively. Five-year disease-free-survival was 90% (95% CI 47.3-98.5). Because of the low number of events, no prognostic factor related to OS and EFS was found. In particular, the MDS variant did not influence the patient's outcome. The median CD3+ cell count on day +30, +90, +180 and +360 were 113, 171, 558 and 1307/mcl, respectively.
Conclusions: These data indicate that TBdepl-haploHSCT is a safe and effective transplant option also in children with MDS. Indeed, the low risk of both non-relapse mortality and acute/chronic GvHD makes this approach particularly attractive in the pediatric setting. Moreover, this haplo strategy compares favorably with T-cell replete approaches [Suo et al., 2020].
Merli: JAZZ: Consultancy; SOBI: Consultancy. Locatelli: Miltenyl: Honoraria; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Bellicum: Consultancy, Membership on an entity's Board of Directors or advisory committees; bluebird bio, Inc.: Consultancy; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal