Abstract
Introduction: Among the genetic lesions described in chronic lymphocytic leukemia (CLL), TP53 and IGHV mutational status are well-established prognostic biomarkers. While mutations resulting in dysregulation of TP53 are associated with chemo-resistance, mutated IGHV (IGHV-M) identifies a good prognosis and unmutated (IGHV-UM) is associated with an aggressive clinical outcome. Thus, molecular assessment of TP53 and IGHV mutational status is recommended to make treatment decisions. Moreover, 30% of CLL patients have a highly homologous complementarity-determining region 3 (CDR3), allowing their classification in subsets based on the stereotypical B-cell receptor immunoglobulins (BcR IG), which have been associated with different clinical features and outcomes. This study aimed to assess the mutational status of TP53 and IGHV and the frequency of stereotypical BcR IG subsets, including CLL#2 and CLL#4 associated with poor and good prognosis, respectively, in a large series of CLL patients in Spain.
Methods: Observational, retrospective, cross-sectional, multicentric study of data from the RED53 project, a collaborative network between the Spanish Group of CLL (GELLC) and Janssen for the characterization of TP53 and IGVH mutational status in naïve CLL candidate patients to receive treatment. Blood samples from 225 institutions were collected between May 2016 and March 2021. Included patients had confirmed diagnosis of CLL and required first-line treatment. Basic demographic variables, leukocyte and lymphocyte counts, and the number of clonal CD5 +/CD19 + lymphocytes were recorded at sample extraction. Clonotypic IGHV-IGHD-IGHJ gene rearrangements and exons 4 to 10 of TP53 were amplified by PCR and sequenced (Sanger). Four analytical reference centers qualified by the European Initiative for CLL (ERIC) determined the mutational status following the ERIC guidelines.
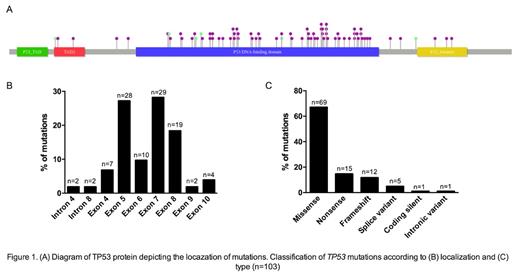
Results: A total of 1097 samples from patients with a median (range) age of 70.0 (27-97) years were analyzed. At sample extraction, patients had a median (range) of 54.5 (2-516) x10 9 leukocytes/mL and 46.1 (0-8810) x10 9 lymphocytes/mL, of which a median (range) of 80.0 (1-100) % (n=754) were clonal CD5 +/CD19 + lymphocytes. The most frequent indications for treatment initiation were progressive/tumoral adenopathy (n=525, 50.4%), progressive lymphocytosis (n=429, 41.2%), cytopenia (n=369, 35.4%), and systemic constitutional symptoms (n=252, 24.2%). Median (IQR) age was 63.0 (55.0, 71.0) years at diagnosis and 70.0 (62.0, 77.0) years at treatment onset. Median (range) time from diagnosis to treatment was 2.7 (0.6-6.1) years. Among 1097 patients, 100 (9.2%) had TP53 mutations with 103 variants, of which only 3 (3.0%) had 2 mutations. Of the 103 mutations, 91 (88.3%), 9 (8.7%), and 3 (2.9%) were pathogenic, likely pathogenic, and of uncertain significance, respectively. Fig. 1 shows mutation localization and type. IGHV was UM in 58% (471/812) and M in 33.1% (269/812) of patients, and unknown/undetermined in 1.8% (15/812), non-productive in 3.2% (26/812), and borderline in 3.8% (31/812) of patients. IGHV rearrangements were undetected in 25.6% (279/1091) of patients and 65.7% (717/1091), 8.5% (93/1091), and 0.2% (2/1091) had 1, 2, and 3 rearrangements, respectively . Of the 30 patients with IGHV3-21 rearrangements, 18 had available data, of which all had CLL#2 subset and, of the 15 patients with IGHV-M, 5 (33.3%) had CLL#2. Minor subsets were found in 7 (46.7%) and 17 (33.3%) of IGHV-M and UM, respectively. The most frequent stereotyped BcR IG subsets were CLL#2, CLL#1, and CLL#6, in 24.3% (18/74), 23% (17/74), and 10.8% (8/74) of patients, respectively. Among the 60 patients with mutated TP53 and IGHV mutational study available, 66.7% (40/60) had IGHV-UM.
Conclusions: In our real-world experience, results regarding TP53 M and IGHV UM (9.2% and 58.0% of patients, respectively) are similar to those reported in previous series of patients requiring first-line treatment, with a slightly higher predominance of IGHV-UM over IGHV-M cases. Subset CLL#2 was the most frequently identified, whereas the frequency of CLL#6 was higher than that reported before. Considering the difficulties associated with the analysis of TP53 and IGHV mutational status of most laboratories diagnosing CLL, the RED53 network allows access to these determinations to naïve CLL patients with active disease by a simple, fast, and standardized method.
Terol: Roche: Consultancy; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel; BMS: Consultancy; Hospital Clinico Valencia: Current Employment. Ferrer Lores: Janssen: Membership on an entity's Board of Directors or advisory committees. Bosch: Roche: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel, Research Funding; AbbVie: Membership on an entity's Board of Directors or advisory committees, Other: Travel; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel; TAKEDA: Membership on an entity's Board of Directors or advisory committees, Other: Travel. Gonzalez Diaz: Astra Zeneca: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Gilead: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Roche: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Janssen: Membership on an entity's Board of Directors or advisory committees, Other: Lectures; Abbvie: Membership on an entity's Board of Directors or advisory committees, Other: Lectures. Crespo: Janssen: Consultancy; Astra Zeneca: Research Funding; Roche/Genentech: Research Funding. Alcoceba: Janssen: Consultancy. Esteve: Janssen: Current Employment. Loriente: Janssen: Current Employment. Villanueva: Janssen: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal