Abstract
Background: FL is an indolent yet incurable disease characterized by recurrent relapses. Pts with R/R FL often have a poor prognosis and limited treatment options, particularly those who have progression of disease within 24 months of frontline treatment (POD24) or are refractory to multiple agent classes.
Glofitamab is a T-cell-engaging, CD20xCD3 bispecific, full-length, 2:1 format antibody with bivalent binding to CD20 (B cells) and monovalent binding to CD3 (T cells). Glofitamab monotherapy with obinutuzumab pretreatment (Hutchings, et al. JCO 2021), or combined with obinutuzumab (Morschhauser et al, Blood 2019; NCT03075696), has shown efficacy and manageable safety in heavily pretreated R/R NHL. Here, we present updated results of glofitamab with three different step-up dosing (SUD) regimens as monotherapy (mono) or combined with obinutuzumab (combo) in R/R FL.
Methods: Obinutuzumab (1000mg) was given 7 days prior to the first dose of glofitamab. For the 3 mono cohorts, intravenous glofitamab SUD was given on Days (D) 1 and 8 of Cycle (C) 1; then at target dose on C2, or as SUD on C1D1, C1D8, C2D1 and target dose on C3D1. For the combo cohort, glofitamab SUD was given on D1 and D8 of C1, then at target dose combined with obinutuzumab 1000mg from C2D1 and onwards (every 21 days for up to 12 cycles). Response rates were based on the Lugano criteria (Cheson et al. J Clin Oncol 2014).
Results: As of May 18, 2021, 53 pts received glofitamab mono SUD (2.5/10/16mg, n=3; 2.5/10/30mg, n=21; 0.5/2.5/10/30mg, n=29) and 19 pts received glofitamab combo SUD (2.5/10/30mg). All pts had FL Grade (Gr) 1-3A (FLIPI 1 high risk score: mono, 28 [53%] pts; combo, 11 [58%] pts). Median age was 64 years (range 33-83) in mono cohorts and 61 years (range 41-78) in the combo cohort; median number of prior therapies was 3 (range 1-12) and 2 (range 1-5), respectively. Twenty-eight (53%) pts in the mono cohorts and 8 (42%) pts in the combo cohort were refractory to last therapy; 16 (30%) and 7 (37%), respectively, were refractory to prior CD20 and alkylating therapy (double refractory). A total of 19 (36%) pts in the mono cohorts and 10 (53%) pts in the combo cohort had experienced POD24.
In the mono cohorts, overall response rate (ORR) was 81% (n=43) and complete metabolic response rate (CMR) was 70% (n=37), with 72% (n=21) CMR in the 0.5/2.5/10/30mg cohort and 67% CMR in both the 2.5/10/16mg (n=2) and 2.5/10/30mg (n=14) cohorts. In the combo cohort, ORR was 100% (n=19) and CMR was 73.7% (n=14).
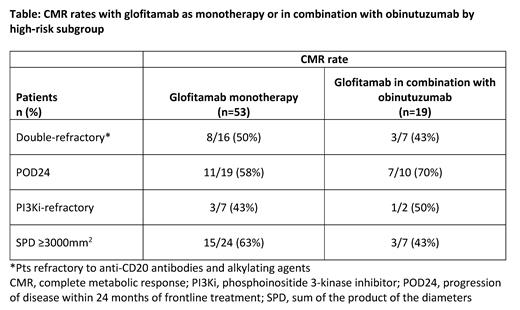
In the mono cohorts 87% (32/37) pts were in CMR, while in the combo cohort 71% (10/14) pts were in CMR. Median follow-up of CMR ranged from 0-14 months (mono) and 0-5 months (combo), and at this cut-off the follow-up is insufficient to assess CMR duration. High CMR rates were observed in high-risk pts (Table).
The most common adverse event (AE) was cytokine release syndrome (CRS): 66% and 79% in the mono and combo cohorts, respectively. CRS rates by mono cohort were as follows: 2.5/10/16mg cohort, 100% (3/3); 2.5/10/30mg cohort, 76% (16/21); 0.5/2.5/10/30mg cohort, 55% (16/29). With mono, CRS events (Lee et al. ASTCT 2019) were mostly Gr 1 (47.2%) and 2 (17.0%); 1 pt had a Gr 3 event (2.5/10/16mg cohort). With combo, 52.6% had Gr 1 CRS and 26.3% had Gr 2 CRS. There were no Gr 3 CRS events in the combo cohort and no Gr 4 or 5 CRS with either regimen. Of pts with CRS, tocilizumab was used to treat CRS in 22.9% pts in mono cohorts and 33.3% pts in the combo cohort. All CRS events were manageable and had resolved at data cut-off. Neurologic AEs were seen in 26 pts (16 mono, 10 combo; 36%); all Gr 1 (n=17) or Gr 2 (n=9). No ICANS-like events related to glofitamab were reported. Other common AEs were infusion related reactions and pyrexia (events separate from CRS; both 28%) and neutropenia (26%) with mono, and neutropenia (58%), anemia (37%) and thrombocytopenia (32%) with combo.
Conclusions: Glofitamab SUD administered as monotherapy or in combination with obinutuzumab achieved high response rates in pts with heavily pretreated R/R FL, including high-risk subgroups. Response rates were comparable to those reported for CAR-T in R/R FL. The safety profile of glofitamab was manageable; CRS events were mostly low grade and occurred mainly in C1 and C2. Considering the short duration of follow-up, additional follow-up is required to further assess the safety and efficacy of glofitamab in this heterogeneously treated population. Further analyses will be presented.
Morschhauser: BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Genentech, Inc.: Consultancy; Genmab: Membership on an entity's Board of Directors or advisory committees; AstraZenenca: Membership on an entity's Board of Directors or advisory committees; Roche: Consultancy, Speakers Bureau; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Servier: Consultancy; Celgene: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees; Epizyme: Consultancy, Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Chugai: Honoraria; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees. Carlo-Stella: AstraZeneca: Honoraria; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Consultancy, Research Funding; ADC Therapeutics: Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Honoraria; Janssen Oncology: Honoraria; Celgene: Membership on an entity's Board of Directors or advisory committees; Karyopharm Therapeutics: Membership on an entity's Board of Directors or advisory committees. Dickinson: Celgene: Research Funding; Takeda: Research Funding; Gilead Sciences: Consultancy, Honoraria, Speakers Bureau; MSD: Consultancy, Honoraria, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Honoraria; Roche: Consultancy, Honoraria, Other: travel, accommodation, expenses, Research Funding, Speakers Bureau; Novartis: Consultancy, Honoraria, Research Funding, Speakers Bureau. Phillips: ADCT, BeiGene, Bristol Myers Squibb, Cardinal Health, Incyte, Karyopharm, Morphosys, Pharmacyclics, Seattle Genetics: Consultancy; AstraZeneca: Consultancy; BMS: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bayer: Consultancy, Research Funding; Incyte: Consultancy, Other: received travel expenses from Incyte, Research Funding. Houot: Bristol-Myers Squibb: Honoraria; CHU Rennes: Current Employment; Roche: Honoraria; Kite: Honoraria; Gilead: Honoraria; MSD: Honoraria; Celgene: Honoraria; Jsnssen: Honoraria; Novartis: Honoraria. Haioun: Celgene: Honoraria, Research Funding; Gilead: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Miltenyi: Honoraria, Research Funding; Servier: Honoraria, Research Funding. Corradini: AbbVie, ADC Theraputics, Amgen, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Honoraria; Incyte: Consultancy; Amgen; Takeda; AbbVie: Consultancy, Honoraria, Other: Travel and accommodations; Novartis; Gilead; Celgene: Consultancy, Other: Travel and accommodations; BMS: Other: Travel and accommodation; Sanofi: Consultancy, Honoraria; KiowaKirin; Incyte; Daiichi Sankyo; Janssen; F. Hoffman-La Roche; Kite; Servier: Consultancy; Novartis, Janssen, Celgene, BMS, Takeda, Gilead/Kite, Amgen, AbbVie: Other: travel and accomodations; AbbVie, ADC Theraputics, Amgen, Celgene, Daiichi Sankyo, Gilead/Kite, GSK, Incyte, Janssen, KyowaKirin, Nerviano Medical Science, Novartis, Roche, Sanofi, Takeda: Consultancy. Hutchings: Novartis: Research Funding; Janssen: Honoraria, Research Funding; Incyte: Research Funding; Genentech: Honoraria, Research Funding; Celgene: Research Funding; Takeda: Consultancy, Honoraria, Research Funding; Roche: Consultancy, Honoraria, Research Funding; Genmab: Consultancy, Honoraria, Research Funding. Sureda: GSK: Consultancy, Honoraria, Speakers Bureau; Sanofi: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Roche: Other: Support for attending meetings and/or travel; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite, a Gilead Company: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Honoraria, Speakers Bureau; BMS/Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Support for attending meetings and/or travel, Research Funding, Speakers Bureau; Bluebird: Membership on an entity's Board of Directors or advisory committees; Mundipharma: Consultancy. Martínez-López: Janssen, BMS, Novartis, Incyte, Roche, GSK, Pfizer: Consultancy; Roche, Novartis, Incyte, Astellas, BMS: Research Funding. Wrobel: Janssen: Honoraria, Speakers Bureau; Roche: Honoraria, Research Funding, Speakers Bureau; BMS: Honoraria, Speakers Bureau; Novartis: Honoraria, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; BeiGene: Honoraria, Speakers Bureau. Lundberg: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Mulvihill: F. Hoffmann-La Roche Ltd: Current Employment, Ended employment in the past 24 months. Perez-Callejo: F. Hoffmann-La Roche Ltd: Current Employment, Current equity holder in publicly-traded company. Relf: Harpoon Therapeutics: Divested equity in a private or publicly-traded company in the past 24 months; F-Star Therapeutics: Current equity holder in publicly-traded company, Divested equity in a private or publicly-traded company in the past 24 months; Roche Pharmaceutical Ltd: Current Employment, Current equity holder in publicly-traded company. Panchal: F. Hoffmann-La Roche Ltd: Current Employment. Humphrey: Roche: Current Employment, Current holder of individual stocks in a privately-held company, Current holder of stock options in a privately-held company. Bachy: Kite, a Gilead Company: Honoraria; Novartis: Honoraria; Daiishi: Research Funding; Roche: Consultancy; Takeda: Consultancy; Incyte: Consultancy.
Glofitamab is a full-length, humanized, immunoglobulin G1 bispecific antibody with a 2:1 molecular format that facilitates bivalent binding to CD20 on B-cells, and monovalent binding to CD3 on T-cells. Glofitamab redirects T cells to engage and eliminate malignant B cells. Glofitamab is an investigational agent. Obinutuzumab (Gazyva) is a CD20-directed cytolytic antibody indicated: in combination with chlorambucil, for the treatment of pts with previously untreated CLL; in combination with bendamustine followed by obinutuzumab monotherapy, for the treatment of pts with FL who relapsed after, or are refractory to, a rituximab-containing regimen; in combination with chemo followed by obinutuzumab monotherapy in pts achieving at least a PR, for the treatment of adult pts with previously untreated stage II bulky, III or IV FL

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal