Abstract
Patients with multiple myeloma (MM) invariably relapse with chemotherapy-resistant disease, underscoring the need for new therapeutic modalities that bypass these resistance mechanisms. FTY720, also known as fingolimod, is an S1P modulator approved by the FDA to treat the relapsing form of multiple sclerosis. Previously we reported that FTY720 exhibits potent anti-myeloma effect in vitro and in vivo in disseminated xenograft model of MM (Beider et al., Clin Cancer Res 2017). Cytotoxic activity of FTY720 was associated with down-regulation of anti-apoptotic protein MCL-1 while not affecting BCL-2 levels. It is therefore conceivable that BCL-2 inhibition using BH3-mimetic venetoclax may improve responses to FTY720.
Incubation of human MM cell lines (n=8) and primary MM cells (n=3) with venetoclax and FTY720 combination synergistically potentiated cell death (CI<0.02), regardless of the MM cells t (11; 14) status. The robust apoptosis induced by venetoclax /FTY720 treatment was accompanied by cytochrome C release, activation of caspase-3 and extensive DNA damage, demonstrated by increased TUNEL staining and elevated levels of phosphorylated histone H2AX, respectively . These effects were associated with down-regulation of BCL-2 protein, stabilization of pro-apoptotic Bak protein, loss of mitochondrial membrane potential, ER stress induction, and inhibition of the AKT/mTOR signaling pathway. Furthermore, the venetoclax /FTY720 combination markedly induced mitochondrial calcium flux and mitochondrial ROS generation.
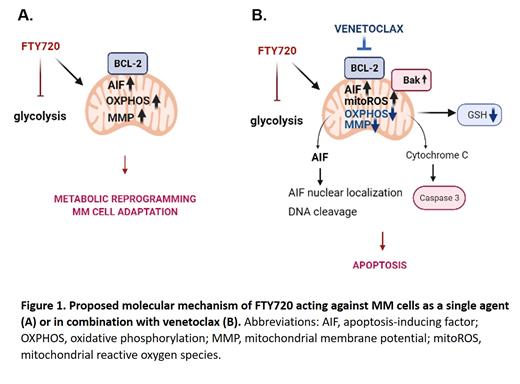
Corresponding with mitochondrial destabilization, venetoclax/FTY720 combination promoted the release of apoptosis-inducing factor (AIF) from the mitochondria to the cytosol and subsequently increased AIF nuclear localization, suggesting its functional role in the execution phase of the apoptosis in response to the dual treatment. AIF is a mitochondrial oxidoreductase that contributes to cell death programs and participates in the assembly of the respiratory chain. Of note, single-agent treatment with FTY720 profoundly up-regulated mitochondrial AIF levels. Given the regulative role of AIF in mitochondrial bioenergetics, we could suggest that increased mitochondrial levels of AIF upon FTY720 exposure may support adaptive responses and promote MM survival upon mitochondrial stress. We thus investigated a possible effect of venetoclax and FTY720 separately or in combination on the metabolic activity of MM cells, observing distinct metabolic profiles of single versus combined exposures. FTY720 significantly suppressed glycolysis, down-regulating the transcript levels of the glycolytic enzymes HK2, PDK1, and LDHA. Glycolytic suppression may result in upregulation of mitochondrial content, which maintains cell survival. In accordance, increased mitochondrial activity was detected in FTY720-treated MM cells, detected by high uptake of MitoSpy Red, a dye that stains mitochondria in a membrane potential-dependent manner. To determine if the changes in the mitochondrial content also altered mitochondrial function, bioenergetic analysis was undertaken. FTY720-treated MM cells demonstrated increased levels of NDUFB8 and UQCRC2 (subunits of mitochondrial respiratory complexes I and III, respectively). Furthermore, FTY720 exposure up-regulated ATP production, suggesting an increase in tumor-protective oxidative phosphorylation (OXPHOS). In agreement, inhibition of mitochondrial electron transport chain using rotenone sensitized MM cells to FTY720, synergistically promoting cell death.
Notably, co-treatment with venetoclax effectively reversed the metabolic changes mediated by FTY720, reducing mitochondrial mass, suppressing mitochondrial activity and strongly down-regulating the pathways related to OXPHOS. Furthermore, venetoclax/FTY720 combination significantly reduced glutathione (GSH) levels, therefore suppressing antioxidative cell responses.
To conclude, we unveil venetoclax role in the metabolic regulation in MM cells. Venetoclax reverses metabolic reprogramming induced by FTY720, suppresses mitochondrial respiration, induces vigorous mitochondrial damage and preferentially targets MM cells in combination with FTY720. These findings may provide the scientific basis for a novel combinatorial anti-myeloma therapy.
Peled: Biokine Therapeutics Ltd: Current Employment; Gamida Cell: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal