In this issue of Blood, Wong et al describe the synthesis and properties of P-G6, a pegylated glycomimetic of the N terminus of P-selectin glycoprotein ligand (PSGL-1), which inhibits the P-selectin–PSGL-1 interaction.1 Consistent with this blockade, P-G6 attenuated human and mouse platelet–monocyte and platelet–neutrophil aggregation in vitro and blocked platelet–leukocyte interactions in vivo. Pegylation endowed P-G6 with a half-life of 16 hours in mice, and a single IV dose of P-G6 inhibited electrolytic injury–induced venous thrombosis in a murine model to the same extent as enoxaparin. Whereas enoxaparin prolonged the bleeding time after tail tip amputation, P-G6 did not. Based on these findings, the authors consider P-G6 an attractive candidate for prevention and treatment of venous thromboembolism (VTE).
There is no question that P-G6 blocks the P-selectin–PSGL-1 interaction, but is this interaction an appropriate target for VTE treatment? Endothelial cells in the deep veins of the leg, particularly those lining the avascular valve cusps, are prone to activation when blood flow is sluggish. Such activation leads to P-selectin expression, which then tethers leukocytes and leukocyte-derived microvesicles onto the endothelial cell surface.2 When activated by proinflammatory cytokines, leukocytes express tissue factor, which initiates thrombin generation and triggers thrombus formation. Leukocytes attach to P-selectin expressed on activated platelets within the thrombus via PSGL-1, where they deliver their profibrinolytic cargo to promote thrombus degradation. Therefore, the catch and slip bond interaction3 between P-selectin and PSGL-1 provides an important link between coagulation and inflammation and contributes to both thrombus formation and degradation.
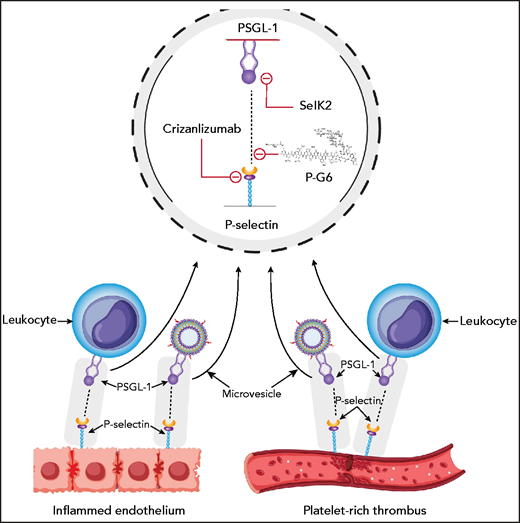
P-selectin on activated endothelium and on activated platelets binds PSGL-1 on leukocytes and leukocyte microvesicles. Crizanlizumab and P-G6 target P-selectin, and SelK2 targets PSGL-1.
P-selectin on activated endothelium and on activated platelets binds PSGL-1 on leukocytes and leukocyte microvesicles. Crizanlizumab and P-G6 target P-selectin, and SelK2 targets PSGL-1.
Wong and colleagues show that P-G6 attenuates venous thrombosis to the same extent as enoxaparin in a murine model. This finding is in keeping with the results of other studies demonstrating that pretreatment with inhibitors of the P-selectin–PSGL-1 interaction (including aptamers, antibodies against P-selectin or PSGL-1, a PSGL-1-Ig chimera, or mimetics of sialyl-Lewis X) attenuated injury or stasis-induced venous thrombosis in murine, feline, or baboon models.4-6 In some models, these inhibitors also appeared to reduce vein wall inflammation and accelerate thrombus resolution, phenomena that might reduce the long-term sequelae of deep vein thrombosis such as the postthrombotic syndrome. Therefore, there is ample evidence that inhibitors of the P-selectin–PSGL-1 axis attenuate venous thrombosis in a variety of animal models, including models in nonhuman primates.
Can the robust results with PSGL-1 blockade in animals be translated to humans? IV GMI-1271, an E-selectin inhibitor, resulted in rapid ultrasound resolution of calf vein thrombosis in 2 patients.7 Capitalizing on these promising findings, SelK2, an inhibitory antibody against PSGL-1, administered IV alone or in combination with enoxaparin, was compared with enoxaparin alone for thromboprophylaxis after elective knee arthroplasty in the COURSE study (https://clinicaltrials.gov/ct2/show/results/NCT03812328?view=results). The primary efficacy outcome was total VTE (the combination of symptomatic VTE and asymptomatic deep vein thrombosis detected by mandatory venography) and the principal safety outcome was the composite of major and clinically relevant nonmajor bleeding. The primary efficacy outcome occurred in 19 of 48 patients (40%) in the SelK2 group, 29 of 61 patients (49%) in the SelK2 plus enoxaparin group, and 17 of 84 patients (20%) in the enoxaparin group. Bleeding occurred in 4%, 2%, and none of the patients in the SelK2-alone, SelK2 plus enoxaparin, and enoxaparin-alone groups, respectively. The 40% and 49% rates of VTE with SelK2 alone or with the combination of SelK2 and enoxaparin are similar to the 48% rate of VTE reported with placebo in such patients.8 Therefore, a strategy aimed at blocking the P-selectin–PSGL-1 axis with a humanized PSGL-1 antibody was ineffective for thromboprophylaxis after knee arthroplasty. However, as an inhibitor of PSGL-1, SelK2 blocks the binding of L-selectin as well as P-selectin. Therefore, the findings with SelK2 may not apply to specific inhibitors of the P-selectin–PSGL-1 interaction such as PG-6 (see figure).
The P-selectin–PSGL-1 axis mediates platelet–endothelial cell, platelet–leukocyte, leukocyte–stromal cell, platelet–leukocyte microvesicle, and platelet–myeloid leukemia cell interaction. Thus, targeting this axis may be of benefit in disorders as diverse as vaso-occlusive crises, postthrombotic syndrome, myeloma, myelofibrosis, and myeloid leukemia.9 Crizanlizumab (Adakveo), an antibody against P-selectin, is approved for prevention of vaso-occlusive crises in patients with sickle cell disease. In the SUSTAIN trial,10 crizanlizumab reduced the incidence of painful crises by 45%. Influenza and pneumonia were the only serious adverse events that occurred in >2 subjects receiving crizanlizumab, but infections were no more common with crizanlizumab than with placebo. Larger trials of crizanlizumab in sickle cell disease are underway, as is a trial in myelofibrosis.
Dimerized P-selectin binding to PSGL-1 can signal via mitogen-activated protein kinase and other pathways. It is likely that P-G6 will inhibit such signaling. In addition to PSGL-1, P-selectin binds ligands such as heparin, von Willebrand factor, and proteins that contain sialyl-Lewis X. It remains to be determined whether P-G6 inhibits P-selectin binding to these partners as well.
P-G6 offers an approach to inhibiting the P-selectin–PSGL-1 axis with high affinity; it will be easier to manufacture than an antibody and is likely to be less immunogenic. If the half-life of P-G6 in humans is like that in mice, once-daily administration via subcutaneous injection may be possible. Therefore, P-G6 needs to be evaluated for a wide variety of indications, including preventing thrombosis in at-risk patients, and in more chronic, harder-to-study conditions, such as the postthrombotic syndrome.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal