In this issue of Blood, Tarlock et al demonstrate that biallelic and single basic-region leucine zipper motif (bZIP) CCAAT enhancer–binding protein α (CEBPA) mutations have an equally favorable prognostic impact in acute myeloid leukemia (AML) in the largest reported series of children, adolescents, and young adults.1
In addition to its size (almost 3000 patients treated on consecutive Children’s Oncology Group protocols), another strength of the study by Tarlock et al is the extensive molecular characterization of almost two-thirds of the patients using next-generation sequencing (NGS); in a fraction of cases, transcriptomic analysis was also performed. NGS allowed analysis of the frequency and prognostic impact of comutations in CEBPA-mutated and -nonmutated groups. An important finding of the study is that the presence of mutations in the cytokine receptor colony-stimulating factor 3 (CSF3R) gene, together with CEBPA biallelic or single bZIP CEBPA mutations, annuls the favorable impact on patient outcomes. Consistent with the equivalent prognosis of CEBPA biallelic and single bZIP CEBPA disease, gene and messenger RNA expression profiles of these 2 groups were comparable and different from those observed in CEBPA wild-type AML cases.
CEBPA is a transcription factor coded by an intronless gene located on chromosome 19. CEBPA mutations are detected in 7% to 15% of AML cases. CEBPA plays a pivotal role in normal granulopoiesis. It binds to regulatory sequences of multiple myeloid genes (CSF3R, IL-6R, GFI-1, KLF1). CEBPA has a carboxyterminal moiety with a bZIP motif. This bZIP motif binds to DNA and other transcription factors. By contrast, the amino-terminal region has 2 transactivation domains.
One-third of CEBPA-mutated AML cases shows a single involved allele, and two-thirds of patients have biallelic mutations.2 On the basis of animal models and translational studies, it was thought that single mutations were insufficient to cause full leukemia. The observation of familial CEBPA leukemias reinforced the 2-hit model of CEBPA. Affected pedigrees usually transmit a single N-terminal mutation and require an additional lesion at the bZIP region for leukemia development.
Studies, mainly in adult patients, have so far supported that only biallelic CEBPA mutations confer improved event-free survival and/or overall survival in AML patients.3-5 On the basis of the favorable prognostic impact of the mutations, the WHO classified CEBPA biallelic–mutated AML as a single clinical/biological entity in 2016.6 European LeukemiaNet and National Comprehensive Cancer Network guidelines include this type of AML in their prognostic classification on the basis of the genetics in the favorable group.7 Given the findings of the Tarlock et al report, should patients with single bZIP CEBPA mutations also be included in the good prognostic category of AML if these findings are confirmed in adults? Should CEBPA-mutated AML with comutations in CSF3R be removed from the favorable risk group? What is the prognosis of patients with biallelic or single bZIP CEBPA mutations with persistent minimal residual disease (MRD)? What is the prognosis of patients with CEBPA-mutated AML with comutations in CSF3A and negative MRD? Can the results of this pediatric and young adult series be extrapolated to adult patients with AML? These are open questions that must be answered in future studies.
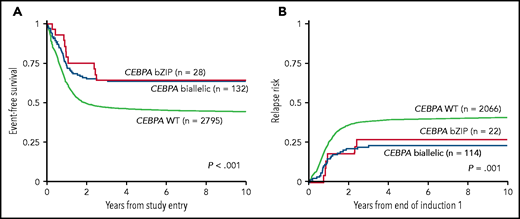
Correlation between patients with CEBPA biallelic, CEBPA single bZIP mutation, and CEBPA wild-type (WT) AML. Event-free survival (A) and relapse (B). See Figure 3A-B in the article by Tarlock et al that begins on page 1137.
Correlation between patients with CEBPA biallelic, CEBPA single bZIP mutation, and CEBPA wild-type (WT) AML. Event-free survival (A) and relapse (B). See Figure 3A-B in the article by Tarlock et al that begins on page 1137.
The treatment recommendation for biallelic CEBPA–mutated AML in first complete remission is that hematopoietic transplantation is not indicated. However, at least 1 report has challenged this recommendation, because it showed better event-free survival in patients with biallelic CEBPA–mutated AML undergoing transplantation.8 However, the retrospective nature of that study, the limited number of patients included, and the consistently good results with intermediate- or high-dose cytarabine consolidation without transplantation justify the chemotherapy approach as the treatment of choice for CEBPA-mutated AML in first complete remission. The data from Tarlock et al indicate that it seems reasonable to extend this approach to cases with single bZIP CEBPA mutations. In contrast, allogeneic transplantation would be an option if comutations in CSF3R were present.
The report presented here highlights the need of investigating comutations in CEBPA-mutated AML. NGS is increasingly being incorporated into routine diagnostic evaluations. Capillary electrophoresis focused on the CEBPA bZIP region combined with CSF3R analysis is another methodology to identify these 2 prognostic genes. Finally, the extended biological characterization of CEBPA-mutated AML is mandatory for introducing molecularly targeted therapies addressing comutations, such as FLT3, IDH1, IDH2, and the CSFR3/JAK/STAT signaling pathway genes. Clinical trials combining these agents with standard chemotherapy and/or novel agents may further improve the outcomes of patients with CEBPA-mutated AML (see figure).
Conflict-of-interest disclosure: J.S. reports personal fees from Astellas, Jazz Pharmaceuticals, AbbVie, Daiichi Sankyo, and Pfizer and grants and personal fees from Novartis, outside the submitted work. J.F.N. reports grants and personal fees from Novartis, outside the submitted work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal