In this issue of Blood, Gauthier et al retrospectively analyzed the outcome of a second infusion of CD19 chimeric antigen receptor (CAR) T cells in patients with B-cell malignancies who relapsed or were refractory to the first infusion. The authors reported durable responses in a significant proportion of patients, with a low incidence of severe toxicity. They also identified actionable pretreatment factors associated with positive outcomes.1
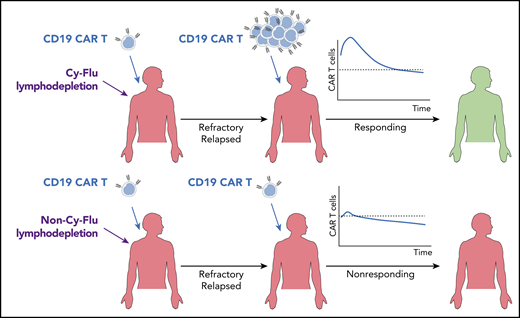
Schematic illustration of the outcomes of a second infusion of CD19 CAR T cells in patients who failed to adequately respond to the first infusion. The addition of Cy-Flu lymphodepletion before the first infusion and an increased CAR T-cell dose for the second infusion are independently associated with durable responses.
Schematic illustration of the outcomes of a second infusion of CD19 CAR T cells in patients who failed to adequately respond to the first infusion. The addition of Cy-Flu lymphodepletion before the first infusion and an increased CAR T-cell dose for the second infusion are independently associated with durable responses.
CD19 CAR T-cell therapy has considerably changed the landscape of treatment options for B-cell malignancies. This therapy was developed as a single infusion of CAR T cells for individuals with relapsed/refractory diseases. However, the frequency of patients who fail to respond or eventually relapse after a partial or complete response is still high. The efficacy of a second infusion in patients unable to achieve durable remissions is still controversial,2,3 and systematic analysis addressing specific clinical and biological factors in this unique setting has been missing.
Gauthier et al here describe the first study entirely dedicated to this topic and including the largest cohort of patients to date (n = 44). Responses were achieved in ∼39% of patients (complete responses, 20%), irrespective of the refractory or relapsed status. However, there were differences across disease types. Despite a higher CAR T-cell dose employed for the second infusion compared with the first, relatively low rates of severe cytokine release syndrome (CRS) and neurotoxicity were reported (9% and 11%, respectively). Multivariable analyses revealed that durable responses were associated with the addition of fludarabine to cyclophosphamide-based (Cy-Flu) lymphodepletion before the first infusion and an increased CAR T-cell dose for the second round of treatment (see figure).
Extensive clinical experience has indicated that CAR T-cell expansion and persistence are required to achieve durable responses. This concept was confirmed here in the setting of a second CAR T-cell infusion. Indeed, Gauthier et al reported that responding patients had higher CAR T-cell peak expansion and longer persistence compared with nonresponding individuals. These results confirm that optimizing these features is mandatory to maximize efficacy. CAR T-cell fitness can be shaped by multiple factors. The first is the intrinsic “quality” of T cells retrieved from patients, which depends on age, tumor histology, and the extent of previous treatments.4 The second parameter is the manufacturing platform, which has evolved over time to promote the enrichment of engineered T cells with stem and central memory phenotypes, endowed with improved proliferative and self-renewal capabilities. Accordingly, responses in chronic lymphocytic leukemia patients were found enriched in gene expression profiles associated with early memory T cells.5 Besides T-cell differentiation, the CD4 and CD8 CAR T-cell composition was also shown to matter. Indeed, the key and synergistic contribution of both subsets in CAR T-cell–mediated responses is becoming clearer. The same group has already shown that formulating the final product at a 1:1 CD4+ to CD8+ CAR T-cell ratio results in superior efficacy and reduced toxicity.2,6 Maintaining this ratio in the product can explain the low rates of severe CRS and neurotoxicity reported in this work. The third crucial aspect modulating CAR T-cell performances is the host environment, including tumor-derived immunosuppressive signals, homeostatic cytokines, and antitransgene immune reactions that may prematurely clear engineered T cells. In this article, Gauthier et al point to the crucial impact of lymphodepleting regimens on the host environment, with critical consequences on CAR T-cell dynamics in vivo. The same team has previously reported that Cy-Flu lymphodepletion was associated with better responses.2,7 Consistently, in this work, they show that even the second round of treatment benefits from applying Cy-Flu before the first infusion. Cy-Flu lymphodepletion resulted in higher CAR T-cell expansion, persistence, and therapeutic efficacy. Besides the potential effect on homeostatic cytokines,8 the data presented suggest that this may be due to a negative impact of Cy-Flu on the priming of endogenous T-cell responses against CAR T cells. The need to avoid premature CAR T-cell clearance may also explain why better therapeutic outcomes were observed with increased CAR T-cell doses. Overall, this study supports the relevance of proper lymphodepleting regimens and fully humanized CAR constructs, especially when planning multiple infusions, which may be the case when treating of solid malignancies.
Understanding why the first infusion failed while the second succeeded in inducing durable remissions can help to design strategies that maximize efficacy. In this work, both CAR T-cell infusions employed cellular products derived from the same leukapheresis, suggesting that differential responses cannot be ascribed to intrinsic T-cell defects. Similarly, the protocol used for CAR T-cell manufacturing was the same, suggesting a negligible role for this aspect in explaining the outcomes. Alternatively, differences in the peculiar host environment and disease status that preceded the 2 infusions may impact the outcomes. Clinical trials designed to address the beneficial effect of a second CAR T-cell infusion should explore this crucial aspect.
Assuming that an allogeneic hematopoietic stem cell transplantation is recommended to consolidate long-term responses, this article shows that a second CAR T-cell infusion may help more patients achieve a favorable outcome. In this scenario, additional clinical factors will potentially impact treatment decisions. Tumor CD19 antigen expression profiling after the first treatment failure may impact the decision about whether to proceed with a second infusion of the same CAR T-cell product or opt for an alternative specificity, such as CD22.6,9
It has been recently reported that infusing CD22 CAR T cells in patients who achieved remission after CD19 CAR T-cell therapy is effective in consolidating responses,10 but a formal comparison of a second infusion of CD19 CAR T cells with CD22 CAR T cells will clarify the cost-benefit ratio of this approach.
In summary, despite its retrospective nature and the small size of the patient cohort for each disease entity, this study will contribute to the design of future clinical trials for heavily pretreated patients who fail first CAR T-cell immunotherapy.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal