Radiation-induced bystander effects (RIBEs) is a neglected, but crucial, area of radiation response. In this issued of Blood, Hu et al1 have provided important new information and mechanistic insights into RIBE-impairment of hematopoietic stem (HSC) and progenitor (HPC) cells in hematopoietic cell transplantation (HCT), with implications for the mitigation of RIBEs.
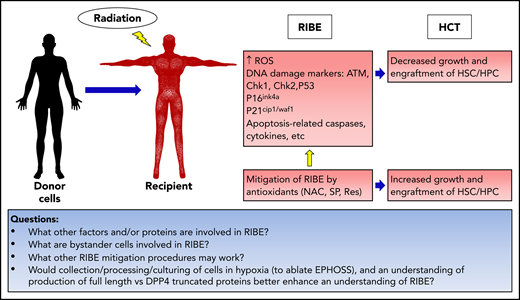
RIBEs on transplanted human HSCs and HPCs in the context of HCT: knowledge and questions remaining.
RIBEs on transplanted human HSCs and HPCs in the context of HCT: knowledge and questions remaining.
Although known, RIBE is not a well-studied area. However, it has significant consequences after exposure of humans, animals, and cells to radiation.2-6 Following up on their studies of negative bystander effects on mouse HSC/HPC,7 Hu et al report the RIBEs on human cells transplanted into irradiated and nonirradiated NOD/Shi-scid/IL-2Rγnull (NOG) mice (see figure). They observed the human transplanted cells in both primary and secondary recipients and demonstrated a significant impairment of HSCs/HPCs in irradiated NOG mouse recipients, using human CD45 chimerism and limiting dilution analysis to calculate the number of SCID-repopulating cells (SRCs; a quantitative measure for human HSCs). They also evaluated how RIBEs influenced the cell cycle, apoptosis, and senescence of human HSCs and HPCs by in vivo and in vitro assessments. They demonstrated that excessive production of reactive oxygen species (ROS) led to HSC/HPC DNA damage associated with upregulation of DNA damage response markers, including ATM, CHK1, CHK2, P53, P16INK4a, P21cip1/waf1 and apoptosis-related caspases. They identified increases from bystander cells of interleukin-1 (IL-1), -6, and -8 and tumor necrosis factor-α, and discuss their roles in context of RIBE impairment of functional populations of HSCs and HPCs. Importantly, they were able to apply this information to mitigate RIBEs in vitro and in vivo by using the following antioxidants: N-acetyl-l-cysteine (NAC), sulforaphane (SF), and resveratrol (Res). NAC, SF, and Res each mitigated RIBEs in vitro and in vivo. In vitro, the antioxidants improved HSC-enrichment, but only SF and Res improved engraftment of HPCs. In vivo, engraftment of HSC-enriched cells was improved by the 3 antioxidants, but only Res improved HPC engraftment (see figure). Although more work is clearly needed, the use of select antioxidants, alone or in combination with other reagents, such as epigenetic modifiers, may be effective in dampening RIBEs and improving clinical HCT. Whether this therapeutic approach is efficacious will have to be determined in human HCT trials. The authors also suggest that mitigation of the adverse effects of RIBEs may have implications for patients who undergo radiotherapy.
That radiation has effects on accessory cells for HPCs was noted by us about 45 years ago.8 We noted that amounts of stimulation (denoted by colony-stimulating activity, years before isolation, identification, purification, and cloning of many different cytokines/chemokines, and other growth factors), when applied to cell cultures, influenced the apparent death of human colony forming cells (CFCs), induced by increasing doses of 137Cs irradiation. These effects were not confined to CFC death, as medium conditioned by cells during in vitro irradiation elicited respective stimulating and inhibitory properties at 600 and 1000 rads. With the current knowledge that there are hundreds of cytokines, chemokines, and other growth-regulating factors, it is time for rigorous identification of the factors produced by accessory cells in the context of RIBEs. Likewise, identification of which accessory cells are involved (eg, monocytes, macrophages, lymphoid cells, other myeloid cell subsets, stromal cells, and other nonhematopoietic cells within the bone marrow microenvironment) is needed (see bottom of figure).
Radiation can be a double-edged sword, having both helpful and detrimental effects.9,10 Every day, we are exposed to background levels of radiation, and this exposure is greatly increased once one leaves earth’s atmosphere. This is problematic for astronauts and is something I became acutely aware of during the 10 years I served as Chairman of the Board of Scientific Counselors and on the Executive Committee of the National Space Biomedical Research Institute (NSBRI, National Aeronautic and Space Administration). Being able to mitigate not only the direct effects of radiation on sensitive tissues and cells such as HSCs and HPCs, but also RIBEs, is crucial for future health and treatment efforts and is not limited to HCT. There are ongoing efforts to prevent RIBEs by using different mediators,11 in addition to those discussed herein.
Several other factors come to mind when evaluating RIBEs on HSCs, HPCs, and other cells affected by radiation. Most studies entail analysis of cells collected and processed ex vivo in ambient air oxygen (∼21% O2) or cultured in ambient air. Ambient air O2 levels are not the same as physoxia (lowered O2 levels present in vivo that are relevant to in vivo physiology). Collecting and processing cells in ambient air results in a phenomenon termed extra physiological shock/stress (EPHOSS).12 EPHOSS is associated with enhanced mitochondrial ROS, and is linked with P53, opening of the mitochondrial permeability transition pore, ROS, hypoxia inducing factor-1α, and the hypoximir miR210. Mitigating EPHOSS by collecting and processing cells at a lowered O2 allows for detection of increased HSCs and decreased slow- cycling HPCs.12,13 Hence, it is appropriate to reevaluate the effects of radiation and RIBEs by collecting/processing and culturing cells at lowered O2/physoxia to remove the confounding influences of EPHOSS on collected cells. This approach will provide a more accurate reading of the results and may be especially important for evaluation of the effects of radiation and RIBEs on cells of aged mice13 and humans.
Another area that could use reanalysis in this context is that of RIBE-induced cytokines, chemokines, and growth factors and whether these molecules, produced or released in response to radiation, are in a full-length or truncated form. Dipeptidylpeptidase4 (DPP-4) is an enzyme that truncates selected proteins with an alanine, proline, or other amino acid at the penultimate N terminus.14 Full-length and DPP-4–truncated proteins do not have similar functional activities. In certain situations, DPP4-truncated proteins (such as granulocyte colony-stimulating factor, granulocyte-macrophage colony-stimulating factor, IL-3, TPO, and EPO) have no or less activity than their full-length forms, but can block activities of their full-length forms.14
In their article, Hu et al add to and bring us closer to a more in-depth analysis of the impact of RIBEs on human HSCs and HPCs in the context of HCT. It is clear that there is much more to be learned about health benefits involving radiation (see bottom of figure). This is a virgin field, ready for continued rigorous evaluation in terms of cell responses and in-depth mechanistic insight.
Conflict-of-interest disclosure: H.E.B. is on the scientific advisory board of Elixell Therapeutics.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal