Abstract
Platelets play significant and varied roles in cancer progression, as detailed throughout this review series, via direct interactions with cancer cells and by long-range indirect interactions mediated by platelet releasates. Microvesicles (MVs; also referred to as microparticles) released from activated platelets have emerged as major contributors to the platelet-cancer nexus. Interactions of platelet-derived MVs (PMVs) with cancer cells can promote disease progression through multiple mechanisms, but PMVs also harbor antitumor functions. This complex relationship derives from PMVs’ binding to both cancer cells and nontransformed cells in the tumor microenvironment and transferring platelet-derived contents to the target cell, each of which can have stimulatory or modulatory effects. MVs are extracellular vesicles of heterogeneous size, ranging from 100 nm to 1 µm in diameter, shed by living cells during the outward budding of the plasma membrane, entrapping local cytosolic contents in an apparently stochastic manner. Hence, PMVs are encapsulated by a lipid bilayer harboring surface proteins and lipids mirroring the platelet exterior, with internal components including platelet-derived mature messenger RNAs, pre-mRNAs, microRNAs, and other noncoding RNAs, proteins, second messengers, and mitochondria. Each of these elements engages in established and putative PMV functions in cancer. In addition, PMVs contribute to cancer comorbidities because of their roles in coagulation and thrombosis and via interactions with inflammatory cells. However, separating the effects of PMVs from those of platelets in cancer contexts continues to be a major hurdle. This review summarizes our emerging understanding of the complex roles of PMVs in the development and progression of cancer and cancer comorbidities.
Introduction
Circulating platelets continuously release platelet-derived microvesicles (PMVs), accounting for as much as ∼70% to 90% of blood-borne MVs in healthy individuals, although PMV plasma concentration also displays a wide range.1 MVs are generally considered to result from outward blebbing at the plasma membrane, entrapping cytosolic components, and carrying them into the extracellular space after membrane scission. Hence, MV membranes mirror those of their cells of origin. Platelets, like most cells, also homeostatically release exosomes, typically smaller diameter vesicles derived from endocytic sorting compartments and released by exocytic fusion of multivesicular bodies with the plasma membrane. These disseminated exosomes are also packed with various platelet-derived molecules, but exosome membranes typically resemble a mix of plasma membrane and internal membrane components.2 Although platelet exosome generation can be enhanced by certain agonists, and exosomes in general are associated with cancer progression in many contexts, the proportion of those that are platelet-derived are not well documented in most cancer studies.3 At present, specific roles for platelet-derived exosomes in cancer progression, distinct from roles of exosomes derived from other cells, are only just beginning to be dissected. However, elevated levels of circulating PMVs are common in the presence of cancer because of enhanced platelet activity via multiple mechanisms (reviewed in Goubran et al4 ), and PMVs therefore constitute a class of cancer biomarker, with PMV concentration potentially correlating with cancer stage.5-7 As PMV generation is driven by sustained calcium-mediated signaling in stimulated platelets over time scales on the order of hours,8 precisely how PMV levels can increase substantially in the face of rapid clearance of activated platelets, as well as typically rapid clearance of PMVs, is still a matter for debate. Nonetheless, PMVs have been a major focus of study in cancer biology and clinical outcomes, in part because of their platelet-like properties.
PMVs resemble their activated platelet forbearers in molecules displayed, both on the surface and in their internal contents, and in this sense, PMVs may be considered to be mediators of many platelet functions. However, PMVs appear to have a selective propensity, distinct from resting platelets, to interact with heterologous cell surfaces via the “activated-platelet”–like surface properties of PMVs. As activated platelets may become trapped in thrombi or other aggregates, PMVs may be more readily available to perform the downstream functions of their platelet progenitors. Another key distinction is that PMVs lack the α and dense (δ) granules of platelets, which harbor a variety of molecules that platelets secrete upon stimulation, with implications in cancer. Hence, roles of platelets, per se, as distinct from PMVs in cancer, may ultimately derive in significant part from selective effects of platelet granular contents. On the other hand, PMVs can reach heterologous cells at sites that can extend beyond the typical reach of platelets, in particular outside the confines of the vascular space, as we and others have observed in tumor microenvironments, as well as external to thrombi, which trap the activated platelets that may have generated those PMVs. This metaplatelet feature of PMVs supports many possibilities for intercellular communication, mediated by PMVs, between platelets and cancer cells or cells in the tumor microenvironment, that could have profound consequences for disease progression as well as for clinical management.9
Ascribing functional roles specifically to PMVs in cancer progression presents multiple challenges (Figure 1). Many of the contributions of platelets to cancer derive from their surface properties, which are mimicked in PMVs, and separation of the relative contributions is still needed in many instances. Technical hurdles to identifying and characterizing PMVs from plasma samples and differentiating PMVs from MVs derived from other cells also continue to confound precise functional assignments. Moreover, important functional drivers, such as surface P-selectin, are not unique to PMVs. Indeed, not only endothelial MVs but also cancer cell–derived MVs often harbor P-selectin, which may itself derive from complex interactions of cancer cells with platelets, endothelium, and PMVs.4 Experimental models, such as PMV depletion and PMV transfusion in tumor-bearing rodents, have provided some clues, but such studies to date are limited, and further translation to understanding of PMV roles in human cancers requires more study.10 The most selective case of PMV depletion in humans, the Scott syndrome,11,12 is sufficiently rare worldwide that cancer cohorts have not been well studied,13 and furthermore, the underlying genetics affects cells other than platelets.14-16 However, there are parallel global and platelet-specific gene deletion mouse models that could provide valuable information regarding the PMV-cancer axis.17
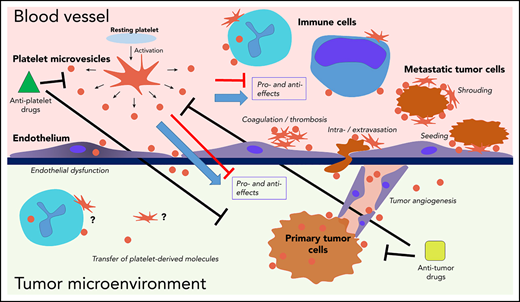
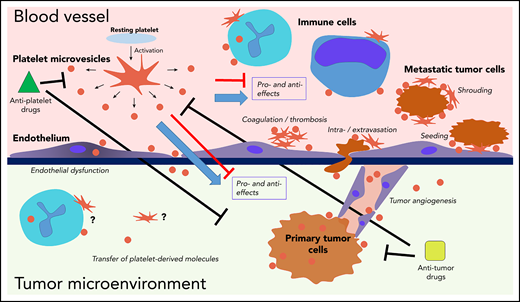
Interactions of platelets and PMVs with mediators and regulators of cancer progression. PMVs have been associated with many similar effects in direct and indirect regulation of cancer progression across many stages. PMVs anchor to many cancer-associated cell types, and they can also be internalized by those cells, supporting transfer of platelet-derived molecules to the target cells. Separating specific effects on cancer from experimental, clinical, and therapeutic standpoints remains a major challenge.
Interactions of platelets and PMVs with mediators and regulators of cancer progression. PMVs have been associated with many similar effects in direct and indirect regulation of cancer progression across many stages. PMVs anchor to many cancer-associated cell types, and they can also be internalized by those cells, supporting transfer of platelet-derived molecules to the target cells. Separating specific effects on cancer from experimental, clinical, and therapeutic standpoints remains a major challenge.
The earliest known and best studied functions of platelets in cancer progression relate to platelet surface properties and their interaction with blood-borne cancer cells, specifically metastatic tumor cells disseminated from primary solid tumors; PMVs can interact with cancer cells in this manner. Platelets and PMVs also interact with endothelium and immune cells in the blood, with profound consequences in cancer that are continually emerging. The following sections summarize the current knowledge and implications of PMVs in tumor metastasis and endothelial and leukocyte interactions, respectively.
PMVs in metastasis and cancer angiogenesis
Roles for platelets in cancer metastasis are well known and reflect several features. Platelets can “shroud” disseminated tumor cells from recognition by natural killer cells, thereby facilitating evasion of host defenses. Platelets promote assembly and stability of the metastatic niche necessary to seed new tumor sites by a combination of angiogenesis driven by platelet-derived chemokines and growth factors and by attracting granulocytes. Platelets can also attract metastasized tumor cells themselves directly to new sites, in part through enhancing tumor cell-endothelial interactions. Moreover, platelets can promote extravasation into the underlying tissue and survival therein.18-22 In addition, platelet-cancer cell interactions potentiate signaling cascades leading to epithelial-to-mesenchymal transition and metastasis.23 The ability of PMVs to support platelet-like functions in each of these aspects of metastasis could reflect the resemblance of PMV surfaces to those of activated platelets. PMV membranes harbor activated platelet integrins, which are other platelet receptors, including those involved in tumor cell interactions such as MHC-1 and P-selectin, which facilitate tumor cell intravasation. Many, but not all PMVs expose phosphatidylserine (PS) on the outer membrane leaflet, which facilitates docking of tissue factor (TF).22,24-27 In contrast, PMVs most likely play little or no role in the effects of activated platelet granular secretion in cancer metastasis, such as transmigration of metastatic cancer cells stimulated by platelet-derived adenosine triphosphate.28,29 Furthermore, the larger surface area and much higher plasma concentration of platelets relative to PMVs seem to make the former better suited to shrouding disseminated metastatic tumor cells, but evidence for or against specific roles of PMVs in this process is lacking. However, although parsing the specific effects of PMVs in cancer metastasis has been challenging, experimental and preclinical evidence indicate that PMVs do have an impact. At the cellular level, PMVs can mediate some of the dysfunction that underlies cancer metastasis, including altered receptor expression.30,31 Recently, PMVs have been shown to transfer CXCR4, a chemokine receptor that induces signaling pathways, promoting tumor cell migration and survival in circulation,32,33 to various solid cancer cell lines.34 PMVs can induce expression of prometastatic proteins, such as metalloproteinases, and promote invasiveness in different lung cancer types, which further supports an increase in metastatic foci in mouse models, suggesting direct in vivo effects of PMVs on intravasation of metastatic tumor cells from the primary site and dissemination to distal sites.35,36 In addition, PMVs promote proliferation of metastatic tumor cells, adhesion to endothelium, and angiogenesis in vitro and after PMV transfusion in rodents,37-40 providing further support for PMVs’ contributing to these functions previously ascribed to platelets. An unexplored area in cancer metastasis is the contributions of PMVs in lymph where they are highly concentrated,41,42 and to lymphangiogenesis, which, like vascular angiogenesis, is driven by growth factors harbored in PMVs.43,44
PMVs in immune cell function and inflammation in cancer
Despite robust literature detailing largely immune-suppressive contributions of MVs in cancer,45 evidence for specific roles of PMVs remains mixed. PMVs have been shown to promote inflammation, as well as to have immunomodulatory effects, all of which may influence host defense in cancer. Because of their presence in circulation and surface expression of platelet-derived antigens, PMVs interact with a variety of immune cells, including directly associating with neutrophils in a calcium-dependent manner via PMV glycoprotein Ib (GPIb) and Mac-1, which is present on neutrophils.46 PMV-neutrophil interactions promote neutrophil activation,47 in part, by PMVs’ transfer of GPIIb and GPIIIa receptors to neutrophils and activation via the NF-κB pathway.48 PMVs can be internalized by neutrophils during inflammatory conditions that may include cancer, although functional outcomes are unclear, and the relevance to inflammation in cancer remains unknown.49 PMVs can bridge interactions between leukocytes, which may potentiate inflammatory responses.50 PMVs produced by collagen-stimulated platelets increase inflammatory cytokine production and are proinflammatory in conditions such as arthritis.51 Whether these PMVs are materially and functionally distinct from those upregulated in the presence of cancer remains to be determined. P-selectin exposed on PMVs, other MVs, or platelets also supports monocyte interaction and activation through the PSGL-1 receptor, but again, direct linkage of PMVs to this process of inflammation in cancer has not been thoroughly explored.52 However, anti-inflammatory effects of PMVs have also been noted, including the modulation of developing macrophages and differentiating dendritic cells toward less reactive states.53 Similarly, PMVs derived from apoptotic platelets (which may or may not be functionally distinct from PMVs derived from activated platelets) promote M2 differentiation of macrophages residing in areas of immune activity, and PMVs can lessen the amount of cytokines and chemokines secreted by these cells, thus supporting immunomodulatory functions of PMVs.54,55 Altogether, the roles of PMVs in immune responses in cancer settings and implications for disease progression and treatment remain ripe for continued investigation.
PMVs in cancer-associated thrombosis
Thromboembolism and disseminated intravascular coagulation (DIC) are strongly associated with cancer and are well documented as significant risk factors for patients with advanced-stage cancer,56-60 with certain cancer types including pancreatic, bladder, and lung cancers exhibiting the highest risks.6,61 Because of their generally procoagulant nature, circulating PMVs have been presumed to play a role in a variety of thrombotic pathologies, including cancers, by increasing the number and density of available sites of prothrombinase complex assembly, including recruitment of a soluble, active-form TF.62,63 One study found PMVs to be 50 to 100 times more potent than thrombin-activated platelets in procoagulant function, with the former harboring increased densities of PS, factor X, and various platelet receptors, including fibrin-binding GPIIb and GPIIIa.64 Plasma TF, most highly concentrated on MV surfaces, is both a prognostic indicator and a driver of venous thrombosis in many cancers.57,58,65-70 However, tumor cell–derived TF+ MVs are clearly major contributors, depending on cancer type as well as cancer stage, and the relative contributions of PMVs are not clear.67-73 For example, pancreatic cancers, which produce high levels of circulating tumor-derived MVs, show strong correlations of TF+ MVs with thrombosis risk and mortality, but PMVs may only support a fraction of the circulating TF.67,68,70,72,74-76 Moreover, in several studies, TF exposed on MVs and overall PS-dependent procoagulant activity in patients with cancer showed association with mortality, but not with the hypercoagulable state or thrombosis, calling into question whether the procoagulant surface properties of MVs, including PMVs, are key drivers of thrombosis risk. However, as in many related studies, methodologies for assessing MVs vary, the relative amounts of active-form TF available may vary, and the causes of mortality may be manifold.67,77-80 Key caveats in all MV studies in patients include different concentrations and functional retention of MVs, depending on the collection technique, the lower detection limit of ∼300 nm vesicle diameter by standard-flow cytometry, which cannot reliably detect the smaller MVs that are likely to be highly relevant in this context,25 and the balance between PS+ and PS− MVs. PS− PMVs may play important roles, but further research is necessary to separate the effects of these PMV classes.81 Newer technologies, such as nanoparticle tracking, support analysis of smaller particles, but also present technical issues. The overall standardization of MV assessment continues to evolve, and the use of orthogonal methods in future studies should help elucidate the functions and mechanisms of PMVs in cancer-associated thrombosis.82-85
Cancer-associated DIC has also been recognized for some time and may also involve contributions from PMVs.86,87 DIC has a more limited presentation in cancer compared with better-studied conditions, such as trauma, leading to the notion that cancer-associated DIC may reflect chronic hypercoagulable state, which may be asymptomatic in early stages of malignancy, but may eventually be marked by depletion of platelets and coagulation factors with localized bleeding adjacent to tumor sites on the one hand and vascular and microvascular thrombosis on the other, as outlined herein.88 MVs, generally TF+ MVs, have been associated with DIC in multiple contexts and particularly in hematologic malignancies89-91 ; but again, whereas increased PMV levels may be associated with DIC in cancer, assignment of specific functional roles and mechanisms awaits further investigation.
Direct experimental evidence demonstrating functional roles of PMVs, specifically in cancer-associated thrombosis in animal models, is still somewhat limited, even though many of these models mimic human disease with respect to PS-dependence and prevalence of TF+ MVs as an apparent major driver of cancer-associated thrombosis.92 Again, PS-compromised/PMV-depleted mouse models, such as those that model Scott syndrome, would be useful tools for testing these functions. Further complicating the issue is evidence of anticoagulant functions of some MVs, including PMVs, such as by exposure of fibrinolytic enzymes of the uPA/tPA systems that may enhance metastasis, but their contributions to cancer-associated thrombosis remain to be clarified.93,94 Nonetheless, anticoagulant treatment such as low-molecular-weight heparin (enoxaparin) reduced venous thromboembolism and prolonged survival in phase 2 clinical trials comprising patients across multiple late-stage cancer types, associated with reduced TF+ MVs.95 Altogether, PMVs have been proposed to be an accessible biomarker for hypercoagulable state and thrombosis risk in cancer patients and may present effective targets for management of thrombosis in cancer patients, but their impact and full mechanisms of action require further study, including more direct functional testing.59,60
PMVs as delivery vehicles of platelet content and as antitumor agents
The platelet-mimicking surface properties of PMVs have generally been associated with cancer-promoting functions. PMVs can also act as delivery vehicles of platelet contents to heterologous cells outside the vasculature that may extend the reach of PMVs beyond that of platelets.96 Such delivery mechanisms, which platelets may also support in some contexts, has been shown to lead to both stimulatory and modulatory effects of PMVs on cancer progression from an experimental perspective. This dichotomy in outcomes reflects the nature of the PMV contents, the target cell type, the relative quantities and concentrations of each, and the experimental context. Many studies of PMV-mediated molecular transfer have focused on transfer of platelet-derived PMV-encapsulated microRNAs (miRNAs) to cells at distal sites, after the markedly high enrichment of miRNAs in platelets and further enrichment and apparently stochastic composition of platelet-derived miRNAs in PMVs, regardless of the inducing platelet agonist.97,98 PMVs were initially shown to transfer platelet-derived miRNAs to endothelium in vitro with effects on gene expression and function in the target cells,99 and subsequent studies have indicated that this function of PMVs may modulate cancer-associated angiogenesis, although outcomes to date have differed, and studies in cancer-specific contexts are mostly lacking. PMV-mediated delivery of platelet miRNA-let-7a to endothelium promoted the angiogenic switch that is necessary for solid tumor growth,100 observed in vitro in human umbilical vein endothelial cells,101 but direct roles in tumor growth in vivo remain to be clarified. Conversely, transfer of miR-96 and -26a by PMVs to the endothelial cells in vitro inhibited their migration and tube formation capacity by downregulating expression of P-selectin and platelet-derived growth factor receptor-α.102 Overall, the effects of PMV-mediated miRNA transfer to vascular and lymphatic endothelia in cancer-associated neovascularization and its contributions to cancer progression require further study in animal models.
An area of emergent interest is PMV interaction with and transferring of platelet content to cancer cells themselves. PMVs, like platelets, can interact with blood-borne leukemic cells and most likely can transfer miRNAs and other molecules, but this phenomenon has not been deeply explored. Although there have been many in vitro studies involving coincubation of PMVs with cancer cell lines from solid tumor types, our group provided some of the first evidence that PMVs can interact with tumor cells in vivo within primary solid tumors and manifest functional effects on cancer progression, specifically via transfer of platelet-derived miRNAs. Reflecting putative special abilities of PMVs, but not resting platelets, to anchor to other cells, PMVs may also harbor a selective propensity to bind to cancer cells. We have shown that PMVs are present in infiltrating human solid tumors of many types, but not in unaffected adjacent tissue, and PMV infiltration is evident across multiple stages and subtypes in lung and colon cancers. Using mouse models of ectopic tumor growth on a background of platelet-specific RNA labeling, as well as transfusion of platelets with labeled RNA into tumor-bearing mice, we demonstrated that PMVs can transfer platelet-derived miRNAs to tumor cells within the primary solid tumor. PMV-mediated transfer of platelet-derived miRNAs led to apoptosis in the tumor cells (in ectopic tumors formed by implantation of Lewis lung carcinoma cells) and suppresses tumor growth. miR-24(-5p) has been identified as a miRNA enriched in the transfer and a key regulator of tumor cell apoptosis and mitochondrial dysfunction, and we identified mitochondrial mt-nd2 among other RNA targets for miR-24 and showed reversal effects on target gene expression, mitochondrial dysfunction, and tumor growth suppression with antagomiR-24 and PMV depletion.10 Thus, PMVs can modulate primary solid tumor growth via transfer of platelet-derived miRNAs and suppression of gene expression in target tumor cells, providing a new antitumor function for PMVs and their resident miRNAs in cancer progression. Translation of these and similar findings into therapeutic approaches, such as engineered PMV mimics as anticancer drug delivery vehicles, is also emerging.103
However, there are multiple considerations in interpreting these findings from molecular cell, pathophysiological, and clinical perspectives.104 Fundamentally, suppression of gene expression by miRNAs is based on the stoichiometry of miRNAs and their target messenger RNAs (mRNAs). PMV levels generally increase as cancer progresses, and delivery of miRNA cargo by the relatively moderate levels of circulating PMVs during the early stages of cancer may result in subclinical suppression of primary tumor growth in patients; certainly, cancer is prevalent worldwide in patients with normal or even elevated platelet count and PMV levels.105 Moreover, growth effects of PMV-mediated molecular transfer of miRNAs to tumor cells may be cancer specific, which again most likely depends in part on the specific miRNAs and mRNA targets involved. In addition, cancer is more often diagnosed in the later stages, making investigating the antitumor properties of PMVs with respect to primary tumor growth in human cancers more difficult. Whether PMVs can modulate the growth of metastatic nodes in a fashion similar to primary tumors is also unknown. We predict that such miRNA-mediated growth suppression would be distinct in individuals, based on cancer stage, as the cancer cells undergo genomic instability and alter gene expression (ie, the mRNA targets of miRNAs) during disease progression. Importantly, platelet miRNA content may also evolve in the presence of cancer.106 Altogether, the molecular, pathophysiological, and clinical manifestations of PMV- and miRNA-mediated tumor growth suppression across cancer types and stages demand more investigation.
In addition to platelet-derived miRNAs, PMVs contain platelet pre-mRNAs and mature mRNAs, proteins, bioactive lipids, secondary signaling molecules, and even mitochondria.7,107,108 Transfer of mitochondria from host cells has been shown to have important roles in the growth and survival of cancer cells, partially driven by the movement of mitochondrial DNA.109,110 As such, transfer of mitochondria and associated DNA may constitute an important role of PMVs in cancer that demands further exploration (specifically, roles of larger PMVs harboring platelet mitochondria, which may be enriched in particular contexts). In addition, PMVs transfer other molecules including mRNAs, small noncoding RNAs other than miRNAs, and secondary signaling messengers such as calcium, all of which may have effects on tumor growth. For example, transfer of platelet-derived TPM-3 mRNA via PMVs to breast cancer cells was recently shown to promote conversion to a metastatic phenotype.111 Notably, although miRNAs are generally abundant in bulk platelet populations,112 young platelets in particular are enriched in megakaryocyte-derived mRNAs.113 However, thrombocytopenia in patients with cancer, which may reflect platelet production defects, ongoing consumption, or side effects of cancer chemotherapy, further complicates our overall understanding of the roles of PMVs as delivery vehicles of platelet molecular contents in cancer contexts.
Summary of the clinical implications of PMVs in cancer
Taking everything discussed herein into account, elevated plasma levels of PMVs in the presence of cancer has various implications that we are still seeking to understand fully. In general, elevated levels of plasma PMVs correlate with advanced-cancer staging and may be indicative of metastasis.7 For example, in one staged study, not only did patients with gastric cancer have elevated levels of circulating PMVs compared with healthy controls, but patients with stage IV disease had increased PMV counts when compared to those with stage I, II, and III disease, indicating that plasma PMV levels may be indicative of metastasis in this cancer type.5 Similarly, PMV levels in patients with myeloproliferative neoplasms were approximately double those in healthy controls,114,115 were increased fourfold in patients with colorectal or oral cancers,116,117 and were up to 10 times higher in patients with breast cancer.118 In each study, the highest PMV levels were seen in more advanced cancers and were typically associated with the presence of distal metastases. Improved cancer outcomes have often been associated with pharmacological suppression of platelet activation, which may reflect, at least in part, interference with the prometastatic effects of PMVs, but the improved outcomes are almost certainly multifactorial. A key example is aspirin, associated with decreased incidence of colorectal cancer and associated mortality,119,120 and hence aspirin is recommended by the US Preventative Services Task Force to be taken daily at low doses to prevent colorectal cancer in healthy individuals.121 In addition to its roles in suppressing platelet activity, aspirin as well as other antiplatelet agents also suppress PMV generation, but whether any effects on PMVs are secondary to those of platelet inhibition in cancer progression is unknown. Moreover, as aspirin also has direct and well-studied antitumor effects, the specific effects of platelet inhibition by aspirin and likely by other antiplatelet drugs on cancer progression, PMV-dependent or otherwise, remain controversial to this day.122-124 Conversely, many commonly used anticancer chemotherapy drugs (for example, inhibitors of the mTOR pathway such as everolimus) also reduce platelet activity and platelet count, thereby inhibiting PMV generation, either directly or indirectly.125 Thus, many confounding issues continue to impede the ability to tease out PMV-specific roles in cancer from clinical data.
From an experimental perspective, selective depletion of PMVs, but not of MVs, from other cells of origin has been achieved in mouse models, such as by deletion of Tmem16f, resembling Scott syndrome, and we have used Par4-deleted mice which had reduced PMVs, as predicted from mechanistic studies.10,17,126 However, as these gene products have complex and varied functions, these models come with substantial caveats for PMV research. Direct targeting of PMVs, both for experimental research and potential clinical use, suffers from many of the same issues as outlined herein; namely, with no specific target sites to differentiate them, the ability to target only PMVs but not platelets remains a formidable challenge. In summary, contributions of PMVs to clinical manifestations of cancer progression and cancer comorbidities and clinical management of both are evident and likely substantial, but remain poorly understood, most fundamentally because of difficulties in separating specific effects of PMVs from those of platelets, or those of MVs derived from other cells. As such, further in vitro and in vivo studies in cancer models, along with focused clinical studies, are needed to elucidate the mechanistic bases of PMVs in each aspect of cancer progression, their clinical implications, and the effects of cancer- or platelet-suppressive treatment strategies on PMV production and their contributions to cancer progression.
Acknowledgment
This work was supported by National Institutes of Health, National Heart, Lung, and Blood Institute grant HL137207 (L.E.G.).
Authorship
Contribution: S.L. and L.E.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lawrence E. Goldfinger, Cardeza Foundation for Hematologic Research, Thomas Jefferson University, 1020 Locust St, Philadelphia, PA, 19107; e-mail: lawrence.goldfinger@jefferson.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal