Background: Treatment options, together with guidelines, for pts with CLL have evolved and expanded with the introduction of novel agents. The informCLLTM registry is the largest US-based prospective, observational registry of pts who received treatment for CLL/SLL in the post-novel agent era. This prospective real-world registry is uniquely positioned to examine the impact of the dynamic treatment landscape, treatment practices, and outcomes in routine clinical setting. Here we present baseline characteristics, prognostic biomarker testing, and treatment patterns for the fully enrolled pt population.
Methods: From Oct 2015 to June 2019, informCLL (PCYC-1134; NCT02582879) enrolled eligible pts with CLL/SLL who were ≥18 years (y), initiated FDA-approved treatment for CLL/SLL within ±45 days of enrollment and provided consent. Pts were classified into 5 groups based on treatment received at enrollment (index): ibrutinib (single agent or combination), chemoimmunotherapy (CIT), chemotherapy (CT), immunotherapy (IT), and other novel agents. Descriptive analyses are presented.
Results: The registry fully enrolled with 1461 pts: 855 (59%) previously untreated and 606 (41%) relapsed/refractory (R/R) pts. Community-based practices enrolled 93% of pts. The median age was 71 y (33% ≥75 y), the majority were male (64%), and 88% had ECOG performance status of 0/1. For pts with staging performed at enrollment (n=852), 51% had Rai stage III/IV. Median (range) time from diagnosis to initial treatment on study was 18.6 months (mos, <0.1−471.3) for previously untreated pts; median time from diagnosis to index treatment was 84.27 mos (1.1−469.4) for R/R pts. Median (range) follow-up for previously untreated pts was 14.9 mos (0.03−46.9) and was 15.3 mos (0.03−44.0) for R/R pts.
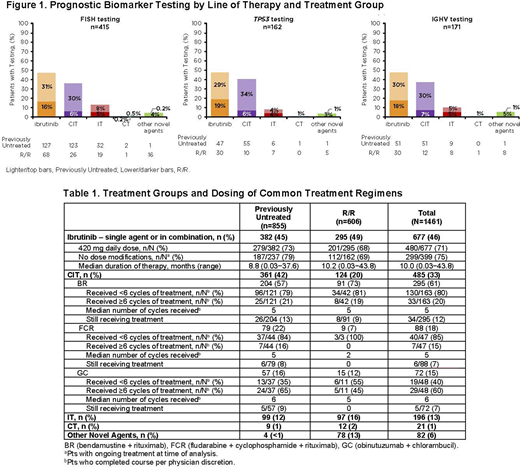
FISH testing was performed in 28% (n=415) of pts and was more frequent in previously untreated vs R/R pts (33% vs 21%). TP53 mutation testing was performed in 11% (n=162) of pts (previously untreated: 13%; R/R: 9%). IGHV mutational status testing was performed in 12% (n=171) of pts (previously untreated: 13%; R/R 10%). Of pts with prognostic biomarker testing, 24% (100/415) had del(17p), 27% (43/162) had TP53 mutation, and 71% (121/171) had unmutated IGHV. Prognostic biomarker testing by treatment group is shown in Figure 1.
Table 1 shows the distribution of pts receiving different treatments on study. Across lines of therapy, the most common treatment was ibrutinib (46%); the majority (87%) started ibrutinib treatment at the recommended daily dose of 420 mg, and pts continuing ibrutinib therapy largely did not require dose modifications (75%). At 12 or 24 mos, 77% (307/401) and 68% (126/184) of pts continued on ibrutinib treatment, respectively. For pts who completed the most common CIT regimens (bendamustine + rituximab [BR] and fludarabine + cyclophosphamide + rituximab), 80% and 85%, respectively, received <6 cycles per physician discretion. Only 6% (n=82) of pts were treated with other novel agents as index treatment; venetoclax (single agent or combination) was the most commonly administered other novel agent, primarily used in R/R (n=56) pts vs previously untreated pts (n=1). Treatment for pts with high-risk features was examined for all pts. Of 100 pts with del(17p), 59% received ibrutinib, 28% CIT, 7% IT, and 6% other novel agents. Of 43 pts with TP53 mutation, 67% received ibrutinib, 21% CIT/CT, 5% IT, and 7% other novel agents. The proportion of pts with del(17p) and/or TP53 mutation receiving CIT decreased over time (2016-2018), although sample size was small. Of 121 pts with unmutated IGHV, 49% received ibrutinib, 39% CIT/CT, 7% IT, and 5% other novel agents.
Conclusions: The informCLL registry provides an opportunity to prospectively assess CLL treatment patterns in the era of novel agents. The most common index treatment was ibrutinib and the majority of ibrutinib-treated pts remained on therapy at 2 y follow-up; CIT (primarily BR) was also used for one-third of patients. Prognostic biomarker testing rates were poor, especially for TP53 and IGHV mutational status. Data from informCLL also indicate a 'knowledge gap' in terms of prognostic marker testing and selection of therapies for pts with high-risk disease. Data from the now complete pt population and with continued follow up will allow for the ongoing evaluation of real-world treatment decisions and pt care that cannot be addressed by data from randomized clinical trials.
Mato:Janssen: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; AbbVie: Consultancy, Research Funding; TG Therapeutics: Consultancy, Other: DSMB, Research Funding; Genentech: Consultancy, Research Funding; BeiGene: Consultancy; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; LOXO: Consultancy, Research Funding. Barrientos:Janssen: Honoraria; Oncternal Therapeutics: Research Funding; Sandoz: Consultancy; Gilead: Consultancy; Genentech: Consultancy; Bayer: Consultancy; AstraZeneca: Consultancy. Sharman:AbbVie: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding; Pharmacyclics: Consultancy, Research Funding; AstraZeneca: Consultancy, Research Funding; Genentech: Consultancy, Research Funding; BeiGene: Research Funding; Bristol Meyers Squibb: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Roche: Consultancy, Research Funding; Acerta: Consultancy, Research Funding; TG Therapeutics: Consultancy, Research Funding. Brander:BeiGene: Other, Research Funding; MEI Pharma: Other, Research Funding; DTRM: Other, Research Funding; AbbVie: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; ArQule: Consultancy, Other, Research Funding; Pfizer: Consultancy, Other; Pharmacyclics LLC, an AbbVie Company: Consultancy, Honoraria, Other, Research Funding; AstraZeneca: Consultancy, Honoraria, Other, Research Funding; Ascentage: Other, Research Funding; Teva: Consultancy, Honoraria; Tolero: Research Funding; TG Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Verastem: Consultancy, Honoraria, Other, Research Funding; NCCN: Other; Novartis: Consultancy, Other; NCCN: Other; Genentech: Consultancy, Honoraria, Other, Research Funding; Juno/Celgene/BMS: Other, Research Funding; Novartis: Consultancy, Other; Teva: Consultancy, Honoraria; Tolero: Research Funding. Kadish:Bluebird Bio: Current equity holder in publicly-traded company; Blueprint Medicines: Current equity holder in publicly-traded company; Teva Pharmaceutical: Current equity holder in publicly-traded company; Bristol-Myers Squibb: Current equity holder in publicly-traded company; Editas Medicine: Current equity holder in publicly-traded company; Johnson & Johnson: Current equity holder in publicly-traded company; Sarepta Therapeutics: Current equity holder in publicly-traded company; AbbVie: Current equity holder in publicly-traded company; Agios: Current equity holder in publicly-traded company; Celgene: Current equity holder in publicly-traded company, Speakers Bureau; Takeda: Speakers Bureau. Arango-Hisijara:Bristol-Myers Squibb: Current equity holder in publicly-traded company; AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Upasani:AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment; Protagonist Therapeutics: Current Employment. Han:Johnson and Johnson: Current equity holder in publicly-traded company; Janssen: Current Employment, Other: Travel expenses. Huang:Janssen Scientific Affairs, LLC: Current Employment; Johnson & Johnson: Current equity holder in publicly-traded company. Iyengar:Express Scripts: Patents & Royalties; AbbVie: Current equity holder in publicly-traded company; Pharmacyclics LLC, an AbbVie Company: Current Employment. Ghosh:Karyopharm: Consultancy; Genmab: Consultancy, Speakers Bureau; SGN: Consultancy, Research Funding, Speakers Bureau; Pharmacyclics LLC, an AbbVie Company: Consultancy, Research Funding, Speakers Bureau; Kite/Gilead: Consultancy, Speakers Bureau; Juno/Celgene/Bristol-Myers Squibb: Consultancy, Research Funding; Janssen: Consultancy, Research Funding, Speakers Bureau; Forty Seven Inc: Consultancy, Other: Research Bureau, Research Funding; Celgene/Bristol-Myers Squibb: Speakers Bureau; AbbVie: Speakers Bureau; AstraZeneca: Speakers Bureau; TG Therapeutics: Consultancy, Research Funding; Roche/Genentech: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal