Introduction
Electronic health records (EHRs), allow use of large clinical databases to inform the practice of medicine. The impact of EHRs on research has been modest due to the lack of validated computable phenotypes for risk factors and outcomes. We developed and validated a computable phenotype for hospital-acquired (HA) venous thrombosis (VTE) to aid future studies about of HA-VTE.
Methods
The study population consisted of all admissions to the medical services (general medicine, cardiology, intensive care unit, and hematology / oncology) between 2010-19 at the University of Vermont (UVM) Medical Center, a 540-bed tertiary acute care hospital. HA-VTE was defined as a deep vein thrombosis of a lower or upper extremity or pulmonary embolism. Data used to develop the computable phenotype include International Classification of Disease (ICD) 9 or 10 discharge codes with the present on admission (POA) flag and current procedure terminology (CPT) codes with dates/times (which include imaging studies). We divided VTE into three groups; upper extremity deep venous thrombosis, lower extremity deep venous thrombosis, and pulmonary embolism, and selected CPT codes which could have diagnosed each VTE group. Our final definition consisted of a VTE ICD code not POA with a VTE site-specific CPT code (but not on the day of admission). We validated the algorithm by randomly abstracting 110 charts in 6 groups; 1) no VTE ICD codes, 2) VTE ICD code POA, 3) VTE ICD code not POA and no CPT code, 4) VTE ICD code not POA and with a CPT code on day 1 of admission, 5) VTE ICD code not POA with a CPT code on day 1 of admission and another 1+ day of admission (this group was incidentally discovered during our initial chart abstraction and validation) and 6) VTE ICD code not POA and with a CPT code after day 1 of admission (our computable phenotype of HA-VTE). We used survey methodology to determine the sensitivity and specificity of our computable phenotype for HA-VTE.
Results
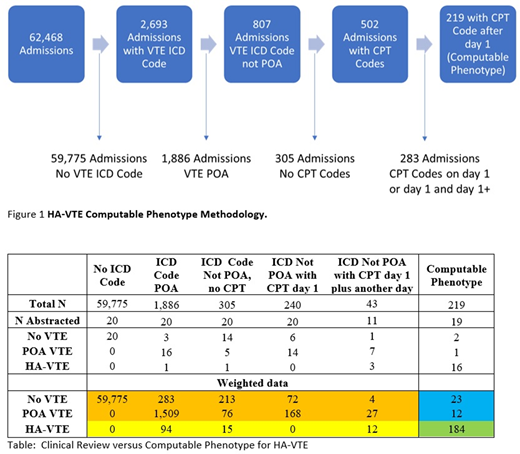
Figure1 shows our methodology and the results from the computable phenotype including how many admissions were analyzed and the total number of HA-VTE identified. For validation we abstracted 110 hospitalizations from the 6 identified groups using a standardized form. The results of the validation by abstraction group are presented in the Table. Using survey methodology, we estimate the incidence of HA-VTE to be 4.9 per 1000 admissions. Among the 20 patients with no VTE ICD code, there were no VTE events. Among the hospitalizations with VTE ICD codes which did not meet our computable phenotype definition of HA-VTE, 5 of 91 individuals had a HA-VTE. One individual had an incorrect POA flag, another had a non-vascular ultrasound which diagnosed the HA-VTE, and 3 admissions with HA-VTE were excluded due to having an imaging study on day 1. Among the 19 patients abstracted for our computable phenotype for HA-VTE, 16 had a HA-VTE. Among the three failures of our HA-VTE computable phenotype, 1 individual had a HA-superficial venous thrombosis (miscoded), the second individual had multiple imaging studies due to a high clinical suspicion, and the third had a VTE on admission (erroneous POA flag). The sensitivity and specificity of our computable phenotype for HA-VTE was 84.2% (CI 78.7-88.9%) and 99.8% (CI 99.77-99.84%).
Conclusions
We developed a computable phenotype for HA-VTE with adequate specificity and excellent sensitivity. This phenotype will be used to assess risk factors for HA-VTE and with appropriate validation to estimate the rates of HA-VTE at other institutions.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal