Introduction
Monoclonal gammopathy of undetermined significant (MGUS) is a premalignant plasma cell disorder that is common in individuals over age 50. MGUS prevalence estimates to date have generally been based on results of serum electrophoresis and immunofixation (for heavy chain [HC] MGUS) and additionally, free light chain (for light chain [LC] MGUS). Mass-spectrometry assays, however, can detect lower levels of monoclonal (M) proteins, identify the isotype and provide accurate quantification of the M-protein. Given the evidence that MGUS is likely present at least 10 years prior to its detection, mass spectrometry may provide more accurate estimates of underlying MGUS. We examined the prevalence of HC-MGUS using matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) mass spectrometry among three risk groups: European Americans (EA), African Americans (AA) and first-degree relatives (FDR) of patients with multiple myeloma (MM), MGUS, chronic lymphocytic leukemia (CLL) or non-Hodgkin's lymphoma (NHL).
Methods
We sampled all AA and a random sample of EA participants ages 50 and older enrolled in the Mayo Clinic Biobank. We also selected unaffected first-degree relatives (limiting to ages 50 and older) from our ongoing family study of hematological malignancies. We screened serum samples by the MALDI-TOF mass spectrometry assay, which is clinically used at Mayo Clinic (known as MASS-FIX) for detection and isotypes of M-proteins. The assay uses isotype-specific nanobody enrichment coupled to MALDI-TOF mass spectrometry. For each of the risk groups, HC- MGUS prevalence was estimated by age (50-59, 60-69, 70+). Age- and sex-adjusted prevalence rates were calculated by direct standardization to the 2010 US population. Ninety-five percent confidence intervals (95% CI) for the prevalence rates and comparisons across the three groups were based on the Poisson distribution. Because free LC information was unavailable, LC-MGUS was not estimated.
Results
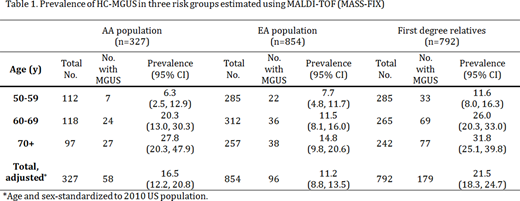
A total 327 AA, 854 EA and 792 FDR were screened for M-proteins using the MASS-FIX assay. All three groups had similar sex (42-44% male) and age distributions (mean[SD] age was 64.5[9.5] yrs., 64.4[8.8] yrs. and 64.9[10.5] yrs. for AA, EA and FDR, respectively). As previously noted using conventional detection methods, the overall prevalence of HC-MGUS was higher in the AA population (16.5% [95%CI: 12.2%, 20.8%]) and FDR (21.5% [95%CI: 18.3%, 24.7%]) than in EA (11.2% [95%CI: 8.8%, 13.5%]), both p-values <0.0001. This pattern was generally seen across age groups (Table 1), with increasing prevalence with age. FDR of MM or MGUS cases had a higher prevalence (22.2% [95%CI: 18.4%, 26.0%]) than FDR of CLL or NHL cases (18.8% [95%CI: 13.3%, 24.4%]), although the difference was not statistically significant (p-value=0.82). Overall prevalence estimates of HC-MGUS using MASS-FIX were at least three-fold higher than estimates using conventional methods alone (i.e. age- and sex-adjusted prevalence in Olmsted County, MN, was 3.1% [95% CI: 2.9%-3.4%]).
Conclusion
We provided some of the first data on prevalence of HC-MGUS across three risk groups, using a sensitive method for detecting monoclonal proteins. The MASS-FIX assay resulted in substantially higher absolute rates of HC-MGUS (at least 3-fold higher) relative to conventional methods. Further, the prevalence of HC-MGUS among the AA and FDR was significantly higher than the EA population. Thus, the more sensitive assay identified greater absolute numbers of individuals with MGUS, but resulted in similar relationships as seen in prior studies across these risk groups. Our results have implications for future studies of the etiology of MGUS and its progression.
Dispenzieri:Janssen: Research Funding; Pfizer: Research Funding; Alnylam: Research Funding; Intellia: Research Funding; Celgene: Research Funding; Takeda: Research Funding. Kumar:Amgen: Consultancy, Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments, Research Funding; Janssen Oncology: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Takeda: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; AbbVie: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Karyopharm: Consultancy; Genecentrix: Consultancy; Cellectar: Other; Carsgen: Other, Research Funding; Merck: Consultancy, Research Funding; Dr. Reddy's Laboratories: Honoraria; Adaptive Biotechnologies: Consultancy; Celgene/BMS: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Genentech/Roche: Other: Research funding for clinical trials to the institution, Consulting/Advisory Board participation with no personal payments; Oncopeptides: Consultancy, Other: Independent Review Committee; IRC member; Kite Pharma: Consultancy, Research Funding; BMS: Consultancy, Research Funding; Tenebio: Other, Research Funding; Sanofi: Research Funding; MedImmune: Research Funding; Novartis: Research Funding. Murray:The Binding Site: Patents & Royalties: Patent Use of Mass Spec to identify monoclonal proteins licensed to The Binding Site.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal