Introduction
Monoclonal immunoglobulin (Ig) is a valuable diagnostic marker in patients with multiple myeloma (MM). An inevitable consequence of extensive Ig synthesis is overload of misfolded proteins that saturate proteasome capacity making the myeloma cells highly sensitive to proteasome inhibitors (PI). Even though PI are regularly used in the clinic, resistance often emerges leaving clinicians with limited treatment options. Therefore, there is a need for a robust marker selecting MM patients for precise PI-based combination therapy.
Methods
We performed a multiple database search for genes associated with Ig production and MM patients' survival. Additionally, we compared gene expression profiles (RNAseq) of primary MM cells with low and high Ig levels. Next, we validated the identified hits by shRNA knockdown and overexpression studies using myeloma cell lines, primary MM samples, and mouse models. We also applied mass spectrometry-based proteomic analysis, advanced biochemical approaches, and genetic models to reveal the Ig production pathway components and function. Finally, we performed a limited rational drug screening to select suitable compounds for combination treatment.
Results
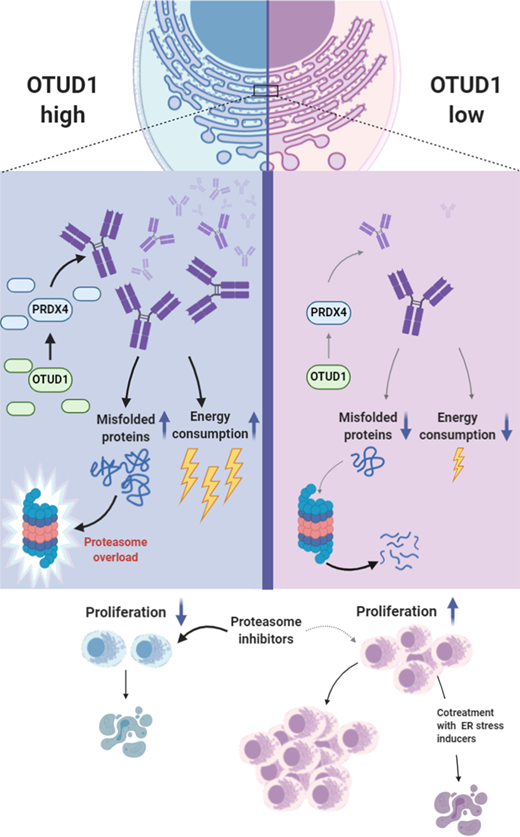
RNAseq and database mining revealed a strong association between the expression of plasma cell-specific deubiquitinase OTUD1, Ig production, and MM patient survival. Suppression of OTUD1 with shRNAs in RPMI8226 and MM1.S cell lines reduced Ig levels, increased proliferation, and induced bortezomib resistance. Conversely, inducible OTUD1 overexpression enhanced Ig production, slowed down proliferation, and increased bortezomib sensitivity. In the xenografts mouse models cells with high OTUD1 levels synthesized more Ig and developed smaller tumors.
Intriguingly, the transcription of Ig genes was not influenced by OTUD1 expression suggesting that OTUD1 functions as a posttranslational regulator of Ig assembly. To gain mechanistic insight into the Ig pathway regulation by OTUD1, we utilized the biotin proximity labeling method (Turbo-ID) combined with mass spectrometry analysis. We found several novel OTUD1 interaction partners including the E3 ubiquitin ligase KEAP1 and endoplasmic reticulum (ER) redox protein PRDX4. We demonstrated that KEAP1 acts upstream of OTUD1 by regulating OTUD1 ubiquitination and stability. Consistently, survival analysis revealed that MM patients with high KEAP1 expression (low OTUD1) had a worse prognosis than patients with low levels of KEAP1 (high OTUD1). PRDX4 regulates disulfite bonds formation during protein folding and is uniquely expressed in fully differentiated plasma cells. Here, we revealed that OTUD1 specifically deubiquitinates and thus stabilizes PRDX4 inside the ER. Additionally, we performed rescue genetic experiments and found a direct link between the OTUD1-PRDX4 axis and Ig production. The increase in OTUD1 expression (high Ig) led to a dramatic increase in the total pool of ubiquitinated proteins formed mainly by misfolded Ig, while OTUD1 knockdown (low Ig) had an opposite effect. We showed that changes in the level of ubiquitinated proteins correlated with PI sensitivity. Of note, OTUD1 did not affect the expression of proteasome subunits, either their enzymatic activity.
Our mechanistic findings prompted us to propose a novel therapeutic opportunity in PI resistant MM patients. We hypothesize that the resensitization of Ig low MM cells to PI could be achieved by enhancing ER stress leading to an increase in misfolded proteins that would ultimately saturate proteasomes. Indeed, from clinically relevant drugs tested so far, the HSP-90 inhibitor (17-AAG) reverted the PI resistance in OTUD1 low (Ig low) myeloma cells. An in vivo validation of the combination treatment and testing of Ig involvement in PI sensitivity and proliferation of MM cells is ongoing.
Conclusion
Here we present the discovery of a novel regulatory mechanism for Ig production in plasma cells. Based on our results and previously published studies, we conclude that Ig synthesis is a clinically significant factor related to PI response and MM patient survival. Our findings suggest that the intracellular Ig level is an important biomarker to identify patients benefiting the most from PI-based therapies. Finally, we provide a rational solution for selective, combination therapy to overcome PI resistance in MM patients with a decreased capacity to synthesize Ig.
Hajek:Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Abbvie: Consultancy, Honoraria; PharmaMar: Consultancy, Honoraria; Oncopeptides: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal