Myeloma precursors (monoclonal gammopathy of unknown significance (MGUS) and smoldering myeloma (SMM)) precede the development of active multiple myeloma. Understanding the genomic and immune mechanisms that underlie transformation to MM may be key to identifying a successful therapy. To this end, we designed a prospective observational study of MGUS/SMM patients to identify genomic, immunological, and clinical parameters that may predict disease progression, and to recognize potential therapeutic targets for MGUS and SMM patients at high-risk of progression.
From December 2015 until April 2019, 132 patients were consented, 100 patients were eligible for the study (41 MGUS, 59 SMM) and included in this analysis. Patients met the current definition of MGUS/SMM per IMWG criteria and were followed at a minimum of every 6 months with standard blood work and urine studies. All patients had advanced imaging/bone marrow biopsy at baseline and after 3 years of follow up. Median follow up time is 24 months (12-48 months). As of 07/01/2020, ten patients (17%, 10/59 SMM and 0%, 0/41 MGUS) progressed to MM and two patients progressed to systemic AL amyloidosis (3%, 2/59 SMM and 0%, 0/41 MGUS). Eight pairs of CD138+ bone marrow samples at baseline and at progression were available and analyzed by flow cytometry using a pre-designed antibody panel. Whole exome sequencing has been performed on 76 samples (matched germline and tumor) from 38 patients (15 MGUS, 17 SMM without progression and 6 SMM that progressed). A total of 24/39 (62%) tumor samples were covered at >100x and 20/37 (54%) germline samples were covered at >50x. After quality control analysis, bulk RNASeq of 144 samples from tumor and microenvironment (TME) cells from 90 patients (38 MGUS and 52 SMM) were included in the analysis.
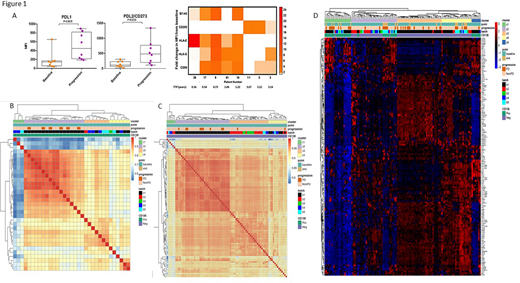
Flow analysis showed upregulation of inhibitory ligands (PD-L1/PD-L2) and B7-H3, CD200, HLA-E, HLA-G and CD59 at progression compared to baseline in 8 paired tumor samples from SMM patients that progressed. (Figure 1A). WES data analysis showed mutations in KMT2C/E (6/38, 4 SMM, 2 MGUS), NRAS/KRAS (5/38, 2 SMM progressors, 1 SMM, 2 MGUS), and FOXO3 (5/38, 1 SMM progressor, 2 SMM, 2 MGUS). Notably, 5/6 mutations in KMT2C/2E were deleterious mutations, all FOXO3 mutations were truncating, and mutations in KMT2C/2E, FOXO3, and NRAS were mutually exclusive. Unsupervised clustering of CD138+ tumor cells RNAseq at baseline identified 3 distinct clusters (C1-C3). All SMM that progressed (n=6) belonged to C2. CD138- TME RNAseq baseline samples were separated into 4 clusters (C1-C4) and 9/11 progressed patients belonged to C2 with distinct expression profiles (Figure 1 panel B/C). Immune deconvolution of TME samples showed lower baseline counts of CD8+ and CD4+ memory resting T cells and higher CD4+ memory activated, gamma delta T cells and dendritic cells in patients with PD (n=11) vs no PD (n=73)(p<0.05). In progressors (n=11), monocytes and NK cells were increased whereas CD4+ naïve, gamma delta T cells, endothelial cells and fibroblasts were decreased at progression compared to baseline (p<0.05). In tumor cells at baseline (n=6 PD; n=27 non-PD), the expression of immune checkpoint genes CD80, CD40, CD70, IL-2, OX40, IFIT1/2/3 was lower and of CCL5 was higher in patients with PD vs stable disease (SD). At baseline in TME cells, PD patients (n=11) had lower expression of immune checkpoint genes CD44, IL7R, CL5 and higher expression of CD276, CD70, CXCL9 compared to non-progressors (n=73) (p<0.05). In progressors (n=11), increased expression of ICOS, CD80, TGFB1, GZMA, GZMB, CD86, HLA-E, HLA-F, CCL5 was observed at progression vs baseline (figure 1 panel D).
Overall, we found extensive changes in the TME composition, in the expression of genes in immune pathways, and in the expression of immune checkpoints, both in tumor and TME samples at baseline and during disease progression. The results of clustering analysis suggest that the features of both tumor and TME at baseline could be possibly used to predict risk of disease progression. Larger studies and validation are needed. Treatment that targets changes in the cell composition and expression of immune checkpoints in myeloma precursor disease may be entertained as a possible therapeutic option.
Manasanch:Novartis: Research Funding; Sanofi: Research Funding; Takeda: Honoraria; JW Pharma: Research Funding; Merck: Research Funding; Adaptive Biotechnologies: Honoraria; GSK: Honoraria; Sanofi: Honoraria; BMS: Honoraria; Quest Diagnostics: Research Funding. Lee:Genentech: Consultancy; Regeneron: Research Funding; Daiichi Sankyo: Research Funding; Sanofi: Consultancy; GlaxoSmithKline: Consultancy, Research Funding; Genentech: Consultancy; Takeda: Consultancy, Research Funding; Janssen: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Amgen: Consultancy, Research Funding. Patel:Cellectis: Research Funding; Janssen: Consultancy, Research Funding; Nektar: Consultancy, Research Funding; Bristol Myers Squibb: Consultancy, Research Funding; Takeda: Consultancy, Research Funding; Oncopeptides: Consultancy; Precision Biosciences: Research Funding; Poseida: Research Funding; Celgene: Consultancy, Research Funding. Kaufman:Janssen: Research Funding; Bristol Myers Squibb: Research Funding; Karyopharm: Honoraria. Bashir:Celgene: Research Funding; KITE: Other: Advisory Board; Amgen: Other: Advisory Board; Purdue: Other: Advisory Board; Takeda: Other: Advisory Board, Research Funding; Acrotech: Research Funding; StemLine: Research Funding. Nieto:Novartis: Other: Grant Support; Astra Zeneca: Other: Grant Support; Affimed: Consultancy, Other: Grant Support; Secura Bio: Other: Grant Support. Qazilbash:Angiocrine: Research Funding; Bioline: Research Funding; Bioclinica: Consultancy; Amgen: Research Funding; Janssen: Research Funding. Berry:Berry Consultants LLC.: Other: Co-owner. Thomas:BMS: Research Funding; Ascentage: Membership on an entity's Board of Directors or advisory committees, Research Funding; X4 Pharma: Research Funding; Xencor: Research Funding; Pharmacyclics: Other: Advisory Boards; Genentech: Research Funding. Orlowski:STATinMED Research: Consultancy; Founder of Asylia Therapeutics, Inc., with associated patents and an equity interest, though this technology does not bear on the current submission.: Current equity holder in private company, Patents & Royalties; Sanofi-Aventis, Servier, Takeda Pharmaceuticals North America, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen, Inc., AstraZeneca, BMS, Celgene, EcoR1 Capital LLC, Forma Therapeutics, Genzyme, GSK Biologicals, Ionis Pharmaceuticals, Inc., Janssen Biotech, Juno Therapeutics, Kite Pharma, Legend Biotech USA, Molecular Partners, Regeneron Pharmaceuticals, Inc.,: Honoraria, Membership on an entity's Board of Directors or advisory committees; Laboratory research funding from BioTheryX, and clinical research funding from CARsgen Therapeutics, Celgene, Exelixis, Janssen Biotech, Sanofi-Aventis, Takeda Pharmaceuticals North America, Inc.: Research Funding. Neelapu:Cell Medica/Kuur: Other: personal fees; Legend Biotech: Other; Calibr: Other; Incyte: Other: personal fees; Bristol-Myers Squibb: Other: personal fees, Research Funding; Merck: Other: personal fees, Research Funding; Kite, a Gilead Company: Other: personal fees, Research Funding; Takeda Pharmaceuticals: Patents & Royalties; Unum Therapeutics: Other, Research Funding; Karus Therapeutics: Research Funding; Novartis: Other: personal fees; N/A: Other; Precision Biosciences: Other: personal fees, Research Funding; Cellectis: Research Funding; Acerta: Research Funding; Allogene Therapeutics: Other: personal fees, Research Funding; Poseida: Research Funding; Celgene: Other: personal fees, Research Funding; Pfizer: Other: personal fees; Adicet Bio: Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal