Introduction
The phase 3 ECHELON-2 study (NCT01777152) demonstrated that frontline treatment with brentuximab vedotin (BV) plus cyclophosphamide, doxorubicin, and prednisone (A+CHP) is superior to cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) for patients (pts) with systemic anaplastic large cell lymphoma (sALCL) or other CD30-expressing peripheral T-cell lymphomas (PTCL) (Horowitz S, et al. Lancet 2019). With a median follow-up of 36.2 months for progression-free survival (PFS), the hazard ratio (HR) (0.71 [95% confidence interval {CI}: 0.54, 0.93], P=0.01) favored A+CHP over CHOP. The median PFS was 48.2 months (95% CI: 35.2, not evaluable) versus 20.8 months (95% CI: 12.7, 47.6) for A+CHP and CHOP, respectively. With a median follow-up of 42.1 months for overall survival (OS), the HR (0.66 [95% CI: 0.46, 0.95], P=0.02) also favored A+CHP over CHOP. Median OS was not reached for either arm. With these results, A+CHP was the first treatment regimen to show an OS benefit over CHOP in this pt population. Herein, we report results with a median follow-up of 44.3 months for PFS and 55.5 months for OS.
Methods
ECHELON-2 is a phase 3, randomized, double-blind, double-dummy, placebo-controlled, active-comparator, multicenter study. Eligible adult pts with previously untreated CD30-positive PTCL (targeting 75% ± 5% with sALCL) were randomized to A+CHP or CHOP for six or eight cycles. Randomization was stratified by histological subtype and international prognostic index score. The primary endpoint of PFS was assessed per blinded independent central review in the primary analysis and per investigator in this updated analysis. Key secondary endpoints were OS, PFS in sALCL, complete remission (CR) rate, and objective response rate (ORR). Subsequent therapies, including BV or BV-containing regimens, were permitted.
Results
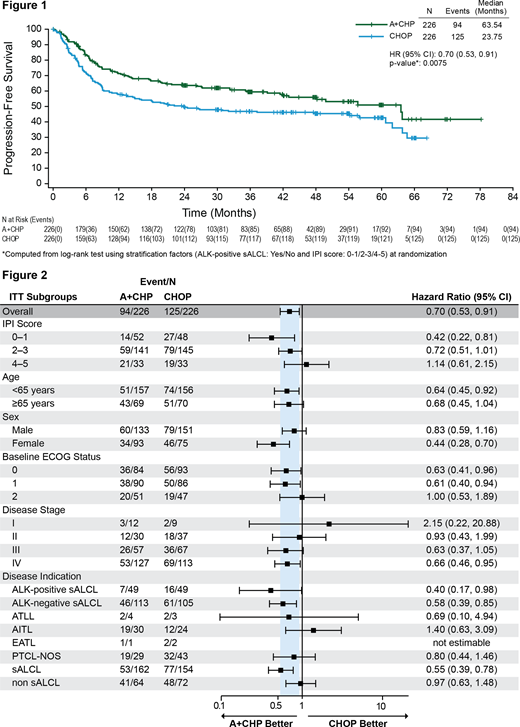
A total of 452 pts were enrolled and randomized 1:1 with 226 pts in each arm. The study included pts with advanced disease (Stage III [27%] and Stage IV [53%]; IPI ≥2 [78%]); given target enrollment, most pts (316 [70%]) had sALCL (218 [48%] anaplastic lymphoma kinase [ALK]-negative and 98 pts [22%] ALK-positive). With additional follow-up, the HRs for PFS per investigator (0.70 [95% CI: 0.53, 0.91], P=0.0075) (Figure 1) and OS (0.74 [95% CI: 0.54, 1.02], P=0.0688) continue to favor A+CHP over CHOP. The median PFS was 63.5 months (95% CI: 42.0, not evaluable) versus 23.8 months (95% CI: 13.6, 55.9) for A+CHP and CHOP, respectively. The estimated 5-year PFS was 50.9% (95% CI: 42.1, 59.1) for the A+CHP arm versus 42.7% (95% CI: 35.3, 49.8) for the CHOP arm. Median OS was not reached for either arm. The estimated 5-year OS was 68.7% (95% CI: 61.3, 75.0) for the A+CHP arm versus 60.3% (95% CI: 52.8, 67.0) for the CHOP arm. The PFS analyses for key prespecified subgroups were generally consistent with the overall study results (Figure 2). In the subset of pts with sALCL, the HR for PFS (0.55 [95% CI: 0.39, 0.78]) also favors A+CHP over CHOP, with an estimated 5-year PFS of 59.8% (95% CI: 48.0, 69.7) for the A+CHP arm versus 48.1% (95% CI: 39.1, 56.6) for the CHOP arm. A total of 23 pts (10%) in the A+CHP arm (16 pts with sALCL, 4 pts with PTCL not otherwise specified, and 3 pts with angioimmunoblastic T-cell lymphoma) and 51 pts (23%) in the CHOP arm received subsequent systemic therapy with BV. In the A+CHP arm, the median time to retreatment was 12.3 months (range, 3, 51); 15 pts (ORR: 65%) had CR (9 pts) or partial remission (6 pts) after retreatment with BV monotherapy (21 pts) or BV-containing regimen (2 pts). With additional follow-up in pts with treatment-emergent peripheral neuropathy (PN) (117 pts A+CHP and 124 pts CHOP), 68% of pts in the A+CHP arm had either resolution or improvement of these events compared with 77% of pts in the CHOP arm. Of the pts with ongoing PN events at last follow-up, 73% in A+CHP arm and 74% in the CHOP arm had grade 1 events, 25% and 23% of pts, respectively, had grade 2 events, and 2% of pts in each arm had grade 3 events.
Conclusions
At 5 years, frontline treatment with A+CHP continues to provide clinically meaningful improvement in PFS and OS versus CHOP, including ongoing remission in ~60% of pts with sALCL, with a manageable safety profile, including continued resolution or improvement of PN.
Additional 5-year results, including data from prespecified subgroups, will be presented.
Horwitz:C4 Therapeutics: Consultancy; Daiichi Sankyo: Research Funding; Affirmed: Consultancy; GlaxoSmithKline: Consultancy; Janssen: Consultancy; Kura Oncology: Consultancy; Miragen: Consultancy; Myeloid Therapeutics: Consultancy; Verastem: Consultancy, Research Funding; ASTEX: Consultancy; Vividion Therapeutics: Consultancy; Beigene: Consultancy; ADCT Therapeutics: Consultancy, Research Funding; Aileron: Consultancy, Research Funding; Celgene: Consultancy, Research Funding; Forty Seven: Consultancy, Research Funding; Infinity/Verastem: Research Funding; Kyowa Hakka Kirin: Consultancy, Research Funding; Millenium/Takeda: Consultancy, Research Funding; Seattle Genetics: Consultancy, Research Funding; Trillium: Consultancy, Research Funding; Corvus: Consultancy; Innate Pharma: Consultancy; Mundipharma: Consultancy; Portola: Consultancy, Research Funding. Pro:Verastem Oncology: Research Funding. Illidge:Takeda: Current Employment, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Iyer:Legend Biotech: Consultancy; Rhizen: Research Funding; Spectrum: Research Funding; CRISPR: Research Funding; Curio Biosciences: Honoraria; Trillium: Research Funding; Target Oncology: Honoraria; Afffimed: Research Funding; Daiichi Sankyo: Consultancy; Merck: Research Funding; Seattle Genetics, Inc.: Research Funding. Advani:Astra Zeneca, Bayer Healthcare Pharmaceuticals, Cell Medica, Celgene, Genentech/Roche, Gilead, KitePharma, Kyowa, Portola Pharmaceuticals, Sanofi, Seattle Genetics, Takeda: Consultancy; Celgene, Forty Seven, Inc., Genentech/Roche, Janssen Pharmaceutical, Kura, Merck, Millenium, Pharmacyclics, Regeneron, Seattle Genetics: Research Funding. Bartlett:BTG: Consultancy; Seattle Genetics: Membership on an entity's Board of Directors or advisory committees, Research Funding; ADC Therapeutics: Consultancy; Forty Seven: Research Funding; Autolus: Research Funding; Acerta: Consultancy; Pfizer: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Immune Design: Research Funding; Janssen: Research Funding; Kite, a Gilead Company: Research Funding; Merck: Research Funding; Millennium: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Consultancy, Research Funding; Affimed Therapeutics: Research Funding; BMS/Celgene: Research Funding; Roche/Genentech: Consultancy, Research Funding. Christensen:Odense University Hospital: Current Employment; Seattle Genetics, Inc.: Research Funding. Morschhauser:Abbvie: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Epizyme: Membership on an entity's Board of Directors or advisory committees; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria; Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Consultancy. Domingo-Domenech:Takeda: Consultancy, Other: Travel, accomodations and expenses , Research Funding; Bristol-Myers Squibb: Other: Travel, Research Funding; Roche: Other: Travel, accomodations and expenses ; Janssen: Other: Travel, accomodations and expenses ; Seattle Genetics, Inc.: Research Funding. Rossi:Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Other: Advisory board; Astellas: Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Sanofi: Honoraria; Jazz: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Pfizer: Membership on an entity's Board of Directors or advisory committees; Alexion: Membership on an entity's Board of Directors or advisory committees; Abbvie: Membership on an entity's Board of Directors or advisory committees. Kim:Donga: Research Funding; Joihnson & Johnson: Research Funding; Kyowa Kirin: Research Funding; Mundipharma: Research Funding; Pfizer: Research Funding; Roche: Research Funding; Takeda: Research Funding; Celltrion: Research Funding. Feldman:Celgene: Honoraria, Research Funding; Cell Medica: Research Funding; Amgen: Research Funding; Kite: Honoraria, Other: Travel expenses, Speakers Bureau; Rhizen: Research Funding; Janssen: Speakers Bureau; Pharmacyclics: Honoraria, Other, Speakers Bureau; AstraZeneca: Consultancy; Bayer: Consultancy, Honoraria; Abbvie: Honoraria; Takeda: Honoraria, Other: Travel expenses; Pfizer: Research Funding; BMS: Consultancy, Honoraria, Research Funding; Trillium: Research Funding; Portola: Research Funding; Corvus: Research Funding; Kyowa Kirin: Consultancy, Research Funding; Eisai: Research Funding; Seattle Genetics, Inc.: Consultancy, Honoraria, Other: Travel expenses, Research Funding, Speakers Bureau; Viracta: Research Funding. Menne:Daiichi Sankyo: Honoraria; Kyowa Kirin: Other: Travel expenses; Pfizer: Honoraria, Other; Roche: Honoraria; Bayer: Other: Travel expenses; Kite/Gilead: Honoraria, Other: Travel expenses; Novartis: Honoraria, Research Funding; Celgene: Honoraria, Other: Travel expenses; Takeda: Honoraria; Atara: Honoraria; AstraZeneca: Research Funding; Amgen: Honoraria, Other: Travel expenses; Janssen: Honoraria, Research Funding. Belada:Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Janssen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Travel expenses, Research Funding; Celgene: Research Funding. Illés:Celgene, Janssen, Novartis,Roche, Takeda: Consultancy; Novartis, Janssen, Pfizer, Roche;: Other: Travel, Accommodations, Expenses; Janssen, Celgene, Takeda, Novartis Pharma SAS, Pfizer Pharmaceuticals Israel, Roche;: Consultancy, Honoraria; Takeda, Seattle Genetics: Research Funding. Tobinai:Daiichi Sankyo: Consultancy, Honoraria; Kyowa Kirin: Consultancy, Honoraria; Bristol-Myers Squibb: Honoraria; Celgene: Consultancy, Honoraria; Mundipharma: Consultancy, Honoraria; Ono Pharma: Consultancy, Honoraria; Solasia: Honoraria; SymBio: Consultancy; Takeda: Consultancy, Honoraria; HUYA Bioscience: Consultancy, Honoraria; Eisai: Honoraria; Yakult: Consultancy, Honoraria; Zenyaku Kogyo: Consultancy, Honoraria; Chugai Pharma: Consultancy, Honoraria. Tsukasaki:Ono Pharma: Consultancy; Mundy Pharma: Honoraria; HUYA: Consultancy, Research Funding; Kyowa Kirin: Honoraria, Research Funding; Celgene: Honoraria; Chugai Pharma: Honoraria, Research Funding; Daiichi Sankyo: Consultancy; Eizai: Research Funding; Seattle Genetics: Research Funding. Yeh:AbbVie: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees; Astellas: Membership on an entity's Board of Directors or advisory committees; Astex: Membership on an entity's Board of Directors or advisory committees. Shustov:Seattle Genetics: Research Funding. Hüttmann:Lead Discovery Center GmbH: Consultancy; Celgene: Honoraria, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Gilead: Honoraria; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: TRAVEL, ACCOMMODATIONS, EXPENSES (paid by any for-profit health care company); Roche: Other: Travel expenses; Seattle Genetics: Research Funding; University Hospital Essen, University of Duisburg-Essen, Essen, Germany: Current Employment. Savage:Verastem: Honoraria; Takeda: Honoraria; Servier: Consultancy; Seattle Genetics: Consultancy, Honoraria, Research Funding; Merck: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy, Honoraria. Zinzani:Roche: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; TG Therapeutics, Inc.: Honoraria, Speakers Bureau; EUSA Pharma: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Merck: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kirin Kyowa: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sanofi: Consultancy, Membership on an entity's Board of Directors or advisory committees; Verastem: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen: Consultancy, Honoraria, Speakers Bureau; Servier: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Incyte: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kyowa Kirin: Consultancy, Speakers Bureau; Eusapharma: Consultancy, Speakers Bureau; Portola: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Immune Design: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Sandoz: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; MSD: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Janssen-Cilag: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celltrion: Consultancy, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Gilead: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Miao:Takeda: Current equity holder in publicly-traded company. Bunn:Seattle Genetics: Research Funding; Takeda: Current Employment. Fenton:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Fanale:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Puhlmann:Seattle Genetics: Current Employment, Current equity holder in publicly-traded company. Truemper:Janssen: Consultancy; Mundipharma: Research Funding; Nordic Nanovector: Consultancy; Roche: Research Funding; Seattle Genetics: Research Funding; Takeda Europe: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal