Introduction
Research in recent years has shown that the class III receptor tyrosine kinase Fms-like tyrosine kinase 3 (FLT3) gene mutation was identified in 30% of Acute Myeloid Leukemia (AML) patients. The FLT3 internal tandem duplication (FLT3-ITD) is positive for 25% of AML cases, while FLT3 tyrosine kinase domain (FLT3-TKD) is present in 5% of cases. Some studies have showed that patients with AML with FLT3 mutation may maintain the alteration in the relapse/refractory (RR) disease, although this is not always the rule. This report is a review about the genetic change of FLT3 mutation in the RR AML scenario.
Methods
It is a retrospective meta-analysis research. A search in June/2020 was performed in PubMed to identify studies with the terms FLT3 (title/abstract) AND Acute Myeloid Leukemia without a restricted date. The papers were select based in title and abstract. Inclusion criteria were adult AML, language in English, Spanish or Portuguese. Exclusion criteria were studies in children and acute promyelocytic anemia. Data were collected from retrospective or prospective studies in which patients with RR AML had the data about FLT3-ITD or FLT-TKD in the diagnosis and relapse.
Results and Discussion
1201 papers were found, in which 688 were excluded by reading the title, and 506 eliminated after the title and abstract revision. From these, 17 were eligible for full text reading. From eligible papers, 6 were rejected. All the studies used PCR as the method of evaluation of FLT3 mutational status. Six studies assessed FLT3 ITD and TKD mutations, four assessed only FLT3-ITD and one checked only FLT3-TKD mutation.
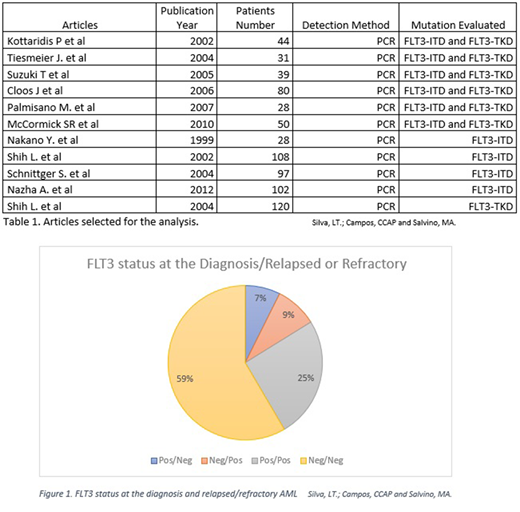
Six articles described mutation status in both FLT3 alterations. The total of 272 patients with RR AML were analyzed in these studies. 74 (27%) were positive for FLT3-ITD at diagnosis and 15 (5%) for FLT3-TKD. In the relapse, 81 (29%) were FLT3-ITD positive and 11 (4%) FLT3-TKD positive. The change in mutation status occurred in 47 (17%) of patients, 3 patients of these change from FLT3-TKD to FLT3-ITD mutation. Three works had patients with acute promyelocytic leukemia, a total of 10 patients. In one work 42 children were included in the study, representing 15% of the total population. Mutational status change from negative to FLT3-ITD in 20 (7%) patients, FLT3-ITD to negative in 14 (5%). From those that were FLT3-ITD positive, 60 (22%) remained positive in the relapse. In patients with FLT3-TKD, change in mutation status from positive to negative occurred in 6 (2%) of the patients, 4 (1%) were negative and gained the mutation, 6 (2%) maintained positive in diagnosis and relapse. 159 (58%) were negative in all the disease course.
When we analyzed only FLT3-ITD mutation, a total of 607 patients were studied at diagnosis and relapsed, 30% were FLT3-ITD positive in relapse. Change in mutational status occurred in 12%. Meanwhile, 392 patients had their mutational status available when considering only FLT3-TKD. 89% were negative in diagnosis and relapsed. 31 (8%) patients had their status changed.
We described FLT3 mutations status in paired samples from diagnosis to relapsed/refractory AML. Our data demonstrate that 41% of patients were positive for FLT3 mutation in some point of the disease. One in six patients had their mutational status altered.
Some patients may have had small clones with mutated cells that were below the limit of the detection by assay and proliferated after treatment. Another hypothesis is that ade novomutation was acquired during the disease course. From the population in the study, 7% were FLT3 positive/negative, which could represent the loss of the mutation post chemotherapy and a selection of a new clone originating from the relapsed disease.
The information about FLT3 status is important due to a possible prognostic value in patients with relapse/refractory disease and because the patient can benefit from a novel therapeutic treatment with FLT3 inhibitors.
Conclusion
FLT3 mutation is an important mutation in AML at diagnosis and relapse/refractory scenario and the mutational status is variable during the disease. Since one in six patients had their mutational status altered in the study, reassessment should be mandatory in any case of relapse and refractoriness. More studies are needed to evaluate the prognostic value of those changes and the development of more sensitive methods could help to find small clones already present in AML at diagnosis.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal