Introduction:
Activation of the T cell Receptor (TCR) by antigen on the surface of an Antigen Presenting Cell (APC) leads to a dramatic change in T cell morphology and formation of a specialized signaling interface with the APC known as the Immune Synapse (IS). The IS allows for controlled cytokine secretion and is an important regulator of T cell activation. IS formation has been found to be dysfunctional in many immune disorders including T cell acute lymphoblastic leukemia (ALL).The actin and microtubule (MT) cytoskeletons have individually been implicated in regulating both IS formation and TCR signaling, but the crosstalk between actin and MTs in T cells has not been well explored. We hypothesized that MACF1 (Microtubule Actin Crosslinking Factor 1), a giant cytoskeleton crosslinker from the spectraplakin family, is present at the IS following Jurkat T cell activation and coordinates actin and MTs. To investigate this, we used TIRFM (Total Internal Reflection Fluorescence Microscopy) and confocal microscopy to image MACF1 organization at the IS.
Methods:
Jurkat E6-1 cells were cultured in RPMI 1640 supplemented with 10% FBS and 1% penicillin/streptomycin. Glass coverslips were coated with Poly-L-Lysine 0.01% and then with 10 µg/mL anti-CD3 for 2 hours at 37 °C to create an activating surface. Cells were dropped carefully and allowed to spread on these coverslips at 37 °C for the indicated time. In the inhibition experiments,5 µM SB415286 or 5 µM PP2 was added to cells at 8 minutes following the initiation of activation and allowed to incubate for an additional 8 minutes.
Cells were fixed with 3.7% paraformaldehyde following activation and then permeabilized with 0.1% Triton X-100. Cells were stained for anti-MACF1, anti-beta tubulin clone KMX-1, respective secondary antibodies, actin-stain 488 phalloidin, and DAPI (4',6-diamidino-2-phenylindole). Fluorescence of labeled cells was imaged using TIRFM with a Nikon Ti microscope and Andor Zyla CMOS camera with a 100x magnification objective (1.49 NA). TIRFM images were analyzed for Corrected Total Cell Fluorescence, which is the total fluorescence of the cell corrected for the background. Mann-Whitney U tests were used for all statistical comparisons. Cells were imaged on Leica SP5X laser confocal microscope with 63x objective for localization of MACF1 with actin and tubulin. Confocal images were deconvolved using the deconvolution lab2 plugin in FIJI (FIJI Is Just ImageJ).
Results:
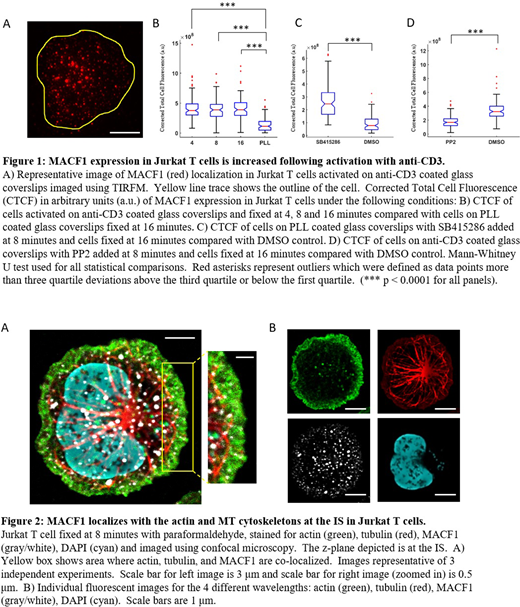
Corrected Total Cell Fluorescence (CTCF) of MACF1 was higher at 4, 8 and 16 minutes following activation compared to the PLL control (Figures 1A and 1B). There was no significant difference in CTCF across the different activation times. To test whether MACF1 is dis-inhibited in Jurkat T cells during activation, we used SB415286 to inhibit Glycogen Synthase Kinase 3-Beta (GSK3B), a protein that inhibits MACF1 function, on PLL-only coverslips. We found MACF1 CTCF to be higher following SB415286 treatment compared to the control, indicating that MACF1 levels increase with GSK3B inhibition (Figure 1C). Thus, SB415286 inhibition of GSK3B mimics T cell activation where Akt upregulation downstream of TCR signaling inhibits GSK3B.
To further test whether CD3 signaling activates MACF1, we stimulated Jurkat cells on anti-CD3 coated glass and then removed the activation signal with the src-kinase inhibitor PP2, which inhibits lymphocyte-specific protein tyrosine kinase (Lck) - an important regulator of early T cell signaling. MACF1 CTCF was lower after PP2 inhibition compared with the control, suggesting that continued CD3 stimulation is necessary for MACF1 accumulation at the IS (Figure 1D).
We then used confocal microscopy to image the spatial distribution of MACF1 and assess whether it localizes with actin and MTs at the IS. We found that MACF1 puncta are present at the F-actin rich lamellipodial region of the IS and appear to localize with MT filaments, suggesting that MACF1 coordinates the two cytoskeleton components at the IS (Figure 2).
Conclusion:
Using high resolution microscopy, we show that MACF1 is upregulated at the IS during Jurkat T cell activation downstream of CD3 signaling through a GSK3B dependent pathway. At the IS, we found the novel localization of MACF1 with actin and MTs. This upregulation and expression of MACF1 during activation warrants more studies of the role MACF1 plays in T cell activation, cytoskeletal dynamics and IS function.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal