Background: Early initiation of graft-versus-host disease (GvHD) is driven by donor alloreactive T-lymphocytes directed against recipient's histocompatibility antigens often overexposed during damage to tissues during the conditioning chemotherapy. Palifermin is a truncated form of human recombinant keratinocyte growth factor (KGF, also known as FGF7) that binds to FGF receptor 2b expressed in many epithelia including the epithelium of the epidermis, oral and GI mucosa, urothelium, and thymus, in which it exerts cytoprotective and regenerative effects. Palifermin also has immunomodulatory effects manifested as improvements in thymic function and downregulation of pro-inflammatory cytokines. Palifermin was FDA approved in 2004 at a dose of 60 mcg/kg/day (x3 consecutive days) for prevention of severe oral mucositis in hematologic malignancy patients receiving autologous hematopoietic stem cell transplant (HSCT). A single dose of 180 mcg/kg/day appears to have similar effects. In animal models, palifermin showed efficacy in controlling acute and chronic GvHD. However, subsequent clinical studies did not confirm efficacy for prevention of GvHD or for stimulation of functional thymus recovery using a dose/schedule based on the one that had been approved for autologous HSCT. We conducted a phase 1 study (NCT02356159) to determine the maximal safe single dose level of palifermin administered prior to starting transplant conditioning.
Methods: This was an open-label, dose escalation study with standard 3+3 design. Four different dose levels of palifermin (180, 360, 540 and 720 µg/kg) were administered as a single dose on day -7 pretransplant. The reduced-intensity conditioning regimen (cyclophosphamide 1200 mg/m2/day IV and fludarabine 30 mg/m2/day IV), was given on days -6 to -3. Sirolimus, tacrolimus and low-dose post-HSCT methotrexate were used for GvHD prophylaxis. On day 0 all subjects received a peripheral blood stem cell (PBSC) graft from an unrelated donor (MUD) matched at least at HLA-A, -B, -C, -DRB1. Prior to transplant, subjects received one or two cycles of disease-specific lymphodepleting induction chemotherapy (EPOCH-F/R or FLAG). Subjects must have been ≥18 years old with a high-risk hematologic malignancy, Karnofsky performance status ≥60%, and acceptable organ function. The primary objective was to assess safety of palifermin and to recommend the phase 2 study dose. DLT was defined as non-relapse mortality before day 30 post-HSCT regardless of attribution to palifermin and non-hematologic grade ≥4 adverse events (AEs) occurring within 14 days after administration. AEs were recorded according to CTCAEv4.
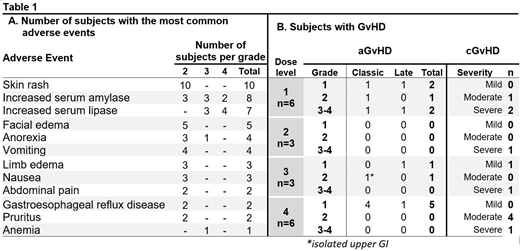
Results: From Oct 2015 to Mar 2019, 18 subjects were enrolled (NHL=7, AML/MDS=5, ALL/LBL=2, CML=2, MPN=1 and MM=1). Kahl's relapse risk was high, standard, and low in 15, 2, and 1 subject, respectively. Median HCT-CI score was 1 (0-4) with median age 47 years (21-66); 14 (78%) were males. Six subjects received dose level 1 (subject #3 was diagnosed with achalasia and grade 4 elevated lipase without radiological signs of pancreatitis, still attributed as DLT possibly related to palifermin). No DLTs occurred afterwards. Three subjects were enrolled onto each of dose levels 2, 3 and 4, with expansion of dose level 4 to 3 more subjects for additional safety exploration. Skin rash and Increased serum amylase or lipase were the most frequent AEs (Table 1A). Grade 3 increased serum amylase and grade 3 possible pancreatitis in subject #3 were the only SAEs attributed to study drug. All subjects engrafted successfully. Day 14 median CD3 and myeloid chimerism was 97% (36-100) and 99% (82-100), respectively, with the median time to 100% CD3 chimerism of 44 days (14-181). Table 1B lists occurrence of GvHD prior to any malignancy relapse. Four subjects had relapsed malignancy, for which 3 received DLI. After a median follow up of 28 months (2-55), 5 subjects have died, 2 malignancy relapses and 3 non-relapse related causes.
Conclusion: Palifermin administered at dose four-times higher than previously given in humans is safe to use in patients undergoing MUD peripheral blood HSCT. MTD was not reached. Recommended phase 2 dose for examining efficacy in prevention of GvHD is 720 µg/kg.
Rubin:NIH/NCI: Ended employment in the past 24 months, Patents & Royalties; KGF/palifermin: Patents & Royalties; Paradigm Shift Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Palifermin was FDA approved at a dose of 60 mcg/kg/day (x3 consecutive days) for prevention of severe oral mucositis in hematologic malignancy patients receiving autologous hematopoietic stem cell transplant (HSCT).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal