Background
Allogeneic Hematopoietic Cell Transplantation (allo HCT) is currently the only curative therapy for high-risk hematologic malignancies due to graft versus tumor effect (GVT), but with the cost of Graft Versus Host Disease (GVHD). Despite extensive research, very few predictors of GVHD have been identified to date.
To study the mechanisms of GVHD and GVT and to identify potential GVHD markers we applied a novel approach, called Transcriptome Fingerprint Assay (TFA), relying on high frequency sampling and blood transcriptome profiling. The TFA is a multiplex microfluidics q-PCR based assay, linked to a computational model for functional analyses, uniquely tailored to answer complex questions on immune perturbations through frequent profiling of gene expression signatures (Chaussabel et a 2014, Speake et a 2017). This approach has been successfully applied to stratify patients' prognosis in autoimmune and infectious diseases. Herein we report the discovery of an association between the expression abundance of a set of genes pre-transplant and the development of acute or chronic GVHD.
Methods
Seventy-eight patients candidate to allo-HCT were enrolled on the study. Patients donated micro-quantities of blood (50-600 microliters) prior to transplant conditioning, weekly until day 100 post-transplant and every 2 weeks thereafter until 2 years after transplant. Detailed clinical, laboratory and therapy annotations are captured during the follow-up. Gene expression of 264 immune-related genes for each sample are measured through Fluidigm BioMark high-throughput qPCR system. Data interpretation is performed through TFA modular analyses and correlated with the clinical annotations. Here we report the univariate analysis of gene expression data and its association with GVHD in 31 patients for whom data are available until at least day 169 post-transplant (169-672, median 513).
SIgnificant genes were tested in a longitudinal analysis with 4 combined linear-mixed models, measuring the parameters: 1) groups (GVHD NO-GVHD) 2) time 3) interaction of groups over time.
Results
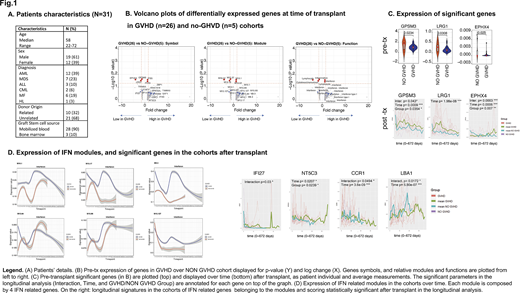
Patients' characteristics are summarized in Fig 1; 26 patients developed GVHD (12 acute GVHD only, 3 late acute GVHD, 1 overlap syndrome, 5 acute and chronic GVHD and 5 chronic GVHD only). By TFA analysis of pre-transplant samples, we identified 3 genes significantly downregulated among patients who developed GVHD (GPSM3, LRG1 and EPHX4), and 2 genes borderline (<1.5 folds) upregulated (IFI35, PLK3). Among these, GPSM3 (an MHC regulated gene) has shown to have alleles protective against severe autoimmune diseases as RA, and LRG1 to facilitate CD34 and myeloid progenitors growth by antagonizing TGF-beta effects, but also to be upregulated in GVHD patients. Several other genes (111) were found to be >1.5 fold upregulated in the GVHD group, but with a non-significant p-value (Volcano-plot Fig.1). Of note, 12 of them were >4 fold upregulated, and their transcriptome pertains to B cells, T lymphocytes, monocytes, IFN, TNF, other cytokines and neutrophil activation. Moreover, among these genes IFN-genes were consistently represented by multiple IFN-modules, emerging as promising candidates.
Longitudinal analyses confirmed that EPHX4 and GPSM3 distinguished the cohorts for all the measured parameters in the post-transplant time. EPHX4 increased over time in both groups but significantly more in the NO-GVHD group (with a fixed effect estimate of 2.05); GPSM3 instead decreased over time, again significantly more in the NO-GVHD group (with a fixed effect estimate of -0.95). LRG3 showed significance only for the parameter "time", resulting after transplant more abundant in the GVHD group, thus confirming previous literature data. IFN modules were also tested longitudinally, showing very distinct patterns in the cohorts, and several significant genes (IFI27, NT5C3, CCR1, LBA1, Fig.1)
Conclusion
Our data demonstrate that TFA may be used to assess expression of immune related genes in transplant patients and to identify specific pathways that may be predictive for GVHD development. Additional work is required to validate our data in a larger cohort and for better understanding of the role of those genes in triggering or protecting against GVHD.
Upon completion of the study and the longitudinal analyses, we aim to define in depth transcriptome signatures of GVHD and relapse.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal