Introduction
The combination of the BCL-2 inhibitor venetoclax with an HMA (HMA/Ven) has improved outcomes in previously untreated patients with AML not eligible for intensive induction therapy. In a phase Ib study, 67% of patients achieved a complete remission (CR) or CR with incomplete recovery of blood counts (CRi) with a median overall survival (OS) of 17.5 months (DiNardo CD et al. Blood 2019; 133(1):7-17). HMA/Ven has also demonstrated efficacy in a heavily pretreated population with relapsed or refractory (R/R) AML, the majority of whom had prior HMA exposure (DiNardo CD et al. Am J Hematol 2018; 93(3):401-7). Measurable residual disease (MRD) is recognized as an independent prognostic indicator important for risk stratification and treatment planning (Schuurhuis GJ et al. Blood 2018; 131(12):1275-91). To date, however, there have been few reports on the effect of HMA/Ven on MRD.
Methods
This is a retrospective case series of patients with AML at a single-center tertiary-care institution. Patients ≥ 18 years of age who were treated with HMA/Ven between January 2017 and June 2020, either in the upfront or salvage setting, for AML were included. Outcomes included CR/CRi rate, MRD response, relapse free survival (RFS), and OS. MRD was assessed via multicolor flow cytometry with a sensitivity of 10-3 (0.1%).
Results
Nineteen patients were identified, 12 (63%) of whom were female. The median age at the time of HMA/Ven initiation was 71 years (range, 21 - 87 years). Ten (53%) patients had de novo AML and 9 had secondary or therapy-related AML. By 2017 ELN criteria, 3 (16%) patients had favorable-risk, 9 (47%) had intermediate-risk, and 7 (37%) had adverse-risk AML. Nine (47%) patients had R/R AML; 5 received HMA/Ven as first salvage therapy, and 4 as 2nd or greater salvage. Three (16%) patients had prior HMA exposure. No patient had prior venetoclax exposure.
Median follow-up was 9.1 months (range, 1-21.1 months). Ten (53%) patients received azacitidine and 9 (47%) were given decitabine. Venetoclax doses ranged from 50 to 400 mg daily, depending on participation in a clinical trial and concomitant medications. Eight patients achieved a CR and 7 patients achieved a CRi for a combined CR/CRi rate of 79%. The CR/CRi rate was 90% (9/10) in the upfront setting, and 66% (6/9) in the salvage setting. The median time and number of cycles to best clinical response was 2.3 months (range, 0.9-3.9 months) and 2 (range, 1-3 cycles), respectively.
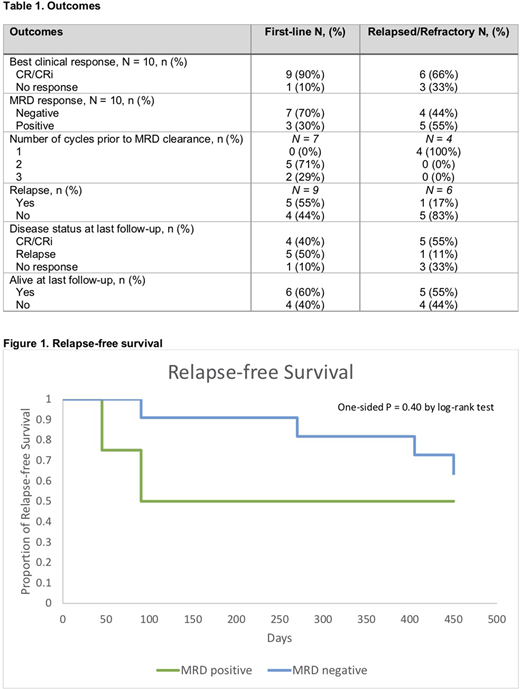
Eleven (73%) of the 15 responders achieved MRD clearance after a median of 2 cycles (range, 1-3 cycles) (Table 1). Two of 4 (50%) MRD-positive patients relapsed, while 4 (36%) of 11 MRD-negative patients relapsed (Figure 1). Relapse occurred at a median of 2.0 months (range, 1.3-2.7 months) in the MRD positive group and 11.0 months (range, 2.8-14 months) in the MRD negative group. One patient died of infectious complications while MRD negative. Three patients, all of whom were treated for R/R disease, proceeded to an allogeneic stem cell transplant (HSCT). Two were MRD negative at the time of HSCT and all remained in remission. At the time of data cutoff, 7 (64%) of 11 MRD-negative patients were alive, and all 4 MRD-positive patients were alive. Causes of death in the MRD-negative group included disease relapse (3 patients) and infection (1 patient). Median overall survival in the entire cohort (range, 32 days-NR) was not reached.
Conclusions
HMA/Ven was highly effective as both upfront and salvage therapy. Surprisingly, the salvage CR/CRi rate in this series was 66%, allowing half of the responders to proceed to HSCT. The majority (73%) of responders achieved MRD negativity. While MRD status influenced RFS, 36% of MRD-negative patients relapsed. Additionally, the same percentage of MRD-negative patients died during follow-up, versus none of the patients with MRD-positivity. This indicates the need for more sensitive methods to assess MRD and for novel therapeutic strategies to eliminate MRD, thereby improving long-term outcomes. Larger prospective studies are needed to define the role of MRD assessment with venetoclax-containing regimens.
Jurcic:AbbVie:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;Celgene:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;Syros Pharmaceuticals:Research Funding;PTC Therapeutics:Research Funding;Arog Pharmaceuticals:Research Funding;Kura Oncology:Research Funding;Forma Therapeutics:Research Funding;Astellas:Research Funding;Genentech:Research Funding;Novartis:Consultancy, Membership on an entity's Board of Directors or advisory committees;Daiichi-Sankyo:Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding;BMS:Consultancy, Research Funding.Campbell:AstraZeneca:Consultancy.Lee:Genentech:Research Funding;Sumitomo Dainippon Pharma Oncology, Inc.:Research Funding;AbbVie:Research Funding;Novartis:Research Funding;Bayer:Research Funding;Celgene:Consultancy;Forty Seven:Research Funding.Heaney:Blueprint Medicines Corporation:Research Funding;BMS:Research Funding;CTI Biopharma:Consultancy, Research Funding;Deciphera:Research Funding;Incyte:Research Funding;Novartis:Consultancy, Research Funding;Sierra Oncology:Research Funding;AbbVie:Consultancy;Partner Therapeutics:Consultancy.Lamanna:Janssen:Consultancy, Membership on an entity's Board of Directors or advisory committees;Octapharma:Research Funding;Juno:Other: Institutional research grants, Research Funding;Gilead:Consultancy, Membership on an entity's Board of Directors or advisory committees;Astra Zeneca:Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding;Pharmacyclics:Consultancy, Membership on an entity's Board of Directors or advisory committees;Genentech:Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding;Bei-Gene:Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding;Abbvie:Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Institutional research grants, Research Funding;Oncternal, Verastem, TG Therapeutics:Other: Institutional research grants, Research Funding;MingSight:Other: Institutional research grants, Research Funding;Loxo:Research Funding;Celgene:Consultancy, Membership on an entity's Board of Directors or advisory committees;Columbia University Medical Center:Current Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal