Background. Aplastic anemia (AA) is a disorder characterized by pancytopenia, hypoplastic bone marrow (BM), and the absence of underlying malignancy. It is believed to be of autoimmune nature. However, some patients fail to respond to the immunosuppressive therapy. The impaired hematopoietic microenvironment could be another reason for BM failure. The severity of AA varies widely from mild, chronic pancytopenia to total hematopoietic failure. The diagnosis of severe (SAA) and non-severe AA (NAA) is based on an absolute neutrophil count as an essential criterion. The aim of the study was to analyze the multipotent mesenchymal stromal cells (MMSC) and fibroblasts colony forming units (CFU-F) in BM of untreated SAA and NAA patients.
Methods. The study included 22 AA patients (8 with SAA and 14 with NAA) in the debut of the disease. In all patients BM was aspirated after informed consent at diagnostic punctures. The proportion of non-hematopoietic CD45-CD34-CD71-CD235-CD90+CD73+CD105+ cells was estimated by FACS. From the BM, MMSC were isolated by the standard method and the concentration of CFU-F was determined. Individual CFU-F-derived colonies were analyzed for their proliferative and differentiation potential. Adipogenic and osteogenic differentiation potential was analyzed with standard techniques. Relative expression level (REL) of several genes had been estimated with RT-PCR in Taqman modification. As a control 19 BM samples of healthy donors of according age were used.
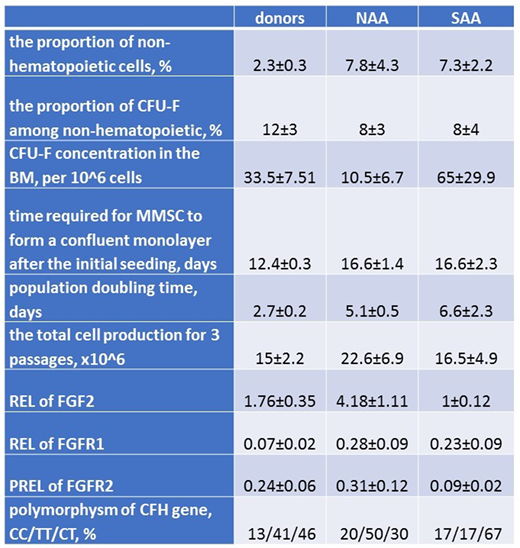
Results. The data are presented in the table. The proportion of non-hematopoietic cells was higher in the BM of AA patients than in healthy donors. We recalculated the proportion of CFU-F among non- hematopoietic cells; it was similar in the BM of AA patients and healthy donors. However, the concentration of -CFU-F was much higher in the BM of patients with SAA then in the BM of patients with NAA. Among NAA patients, 2 had PNG clone and unlike other NAA patients increased CFU-F concentration, comparable to patients with SAA. It seems that the character of stromal cell damage depends on the severity of AA. Individual CFU-F- derived clones from the BM of NAA patients had very limited proliferative potential, while those of SAA patients did not differ from colonies of healthy donors. The analysis of CFU-F-derived colonies differentiation ability revealed that the proportion of the precursors that did not respond to the differentiation induction was higher in the BM of AA patients than in donors. It reflects the involvement of a certain subpopulation of stromal precursors that are either pre-differentiated into fibroblasts, or, conversely, earlier precursors of the hematopoietic microenvironment, which were not able to differentiate into osteoblasts and/or adipocytes within standard time.
The analysis of the MMSC growth characteristics revealed that the time required for MMSC from SAA and NAA patients to form a confluent monolayer after the initial seeding and the population doubling time, were significantly higher than in MMSC of healthy donors. Thus, the proliferation rate of MMSC of AA patients is reduced. Nevertheless, the total cell production for 3 passages did not differ in cultures of AA patients and healthy donors. Therefore, the proliferative potential of MMSC of AA patients is not altered. Probably MMSC being analyzed ex vivo can restore their function. However, the analysis of REL of genes regulating the proliferation (FGF2, FGFR1, FGFR2) in MSCs had revealed the differences in comparison with donors and between SAA and NAA. Moreover, the analysis of the polymorphism in CFH gene, participating in immunomodulation, showed that the distribution differs between NAA and SAA patients.
Conclusions. Stromal precursors in BM of untreated NAA and SAA patients are impaired and differ between the two subtypes of AA. It seems that the differences between NAA and SAA may lay not only in the absolute neutrophil count but also in the BM stroma itself. This effect could participate in the pathogenesis of AA or be the consequence of compensatory reaction of stromal microenvironment to the hematopoiesis failure.
This work was supported by the Russian Foundation for Basic Research, project no. 19-015-00280.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal