In this issue of Blood, Di Giandomenico et al have used a megakaryocyte (MK)-specific tgfb1 knockout strategy to identify an unsuspected physiological role of MKs in regulating steady-state erythropoiesis by restraining progenitor cell and erythroblast (ERB) production.1
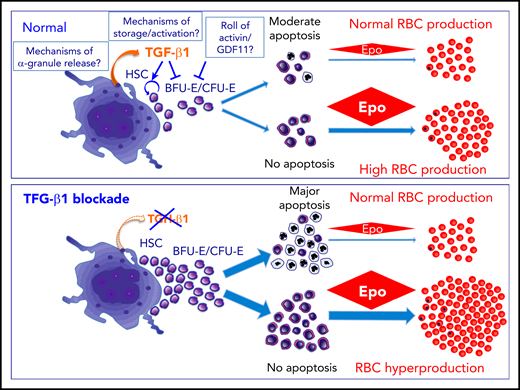
In homeostatic conditions, MKs via TGFβ1 restrain stem cells and erythropoiesis with moderate ERB apoptosis that can be prevented by EPO to increase RBC production. Genetic or pharmacologic blockade of TGFβ1 awakens quiescent stem cells and unleashes erythropoiesis but in an ineffective way with major apoptosis. EPO administration may prevent apoptosis and lead to major RBC production. Many questions remain to investigate in order to fully understand this regulatory process.
In homeostatic conditions, MKs via TGFβ1 restrain stem cells and erythropoiesis with moderate ERB apoptosis that can be prevented by EPO to increase RBC production. Genetic or pharmacologic blockade of TGFβ1 awakens quiescent stem cells and unleashes erythropoiesis but in an ineffective way with major apoptosis. EPO administration may prevent apoptosis and lead to major RBC production. Many questions remain to investigate in order to fully understand this regulatory process.
Transforming growth factor β1 (TGFβ1) is the major member of a superfamily of cytokines that is encoded by 33 genes involved in cell survival, proliferation, and differentiation. They are classified in 4 main families: BMP, GDF, activins, and TGFβ. They have multifunctional roles in most cellular systems through binding to 2 types of cell-surface serine-threonine kinase receptors, known as type I and type II receptors, that lead to the activation of transcription factors of the SMAD family. They behave as regulators of inflammatory and reparative responses and are involved in several diseases.2
Using in vitro approaches, it has been shown that the TGFβ superfamily plays an important role in the regulation of hematopoiesis at different cellular levels, including hematopoietic stem cell (HSC) cell cycle, cell fate, and differentiation, more particularly for the erythropoietic and megakaryocytic lineages. The in vivo role of TGFβ1 for hematopoiesis has been difficult to assign because mice constitutively lacking Tgfb1 died early after birth from an inflammatory disease. However, by this approach it was still possible to show that TGFβ1 plays a central role in the function of HSC.3
In the bone marrow, MKs are the main cellular sources of TGFβ1, which is also synthesized by several immune cells, macrophages, and bone marrow stromal cells. The major role of the MK-derived TGFβ1 on HSC quiescence has been demonstrated by diphtheria toxin–induced MK ablation. This ablation leads to a 74% decrease in TGFβ1 protein associated with induction of HSC proliferation. However, MK-derived CXCL4 (PF4) also controls HSC quiescence. Therefore, Zhao et al could directly demonstrate the role of MK-derived β1 by a specific tgfb1 ablation in MK.4 Di Giandomenico et al, using the same so-called tgfb1ΔMk/ΔMk model, have extended this approach to erythropoiesis.
Suppression of TGF-β1 production by MKs expands erythroid progenitors and ERBs. However, ERBs undergo apoptosis, and no excess of red blood cells (RBCs), or other blood cells, was observed. Similarly, blockade of TGFβ1 activity with an antibody amplified the early erythroid progenitor pool resulting in apoptotic ERBs. In both models, supplementation by erythropoietin (EPO) triggered RBC production by rescuing apoptotic ERBs. These results are in agreement with data showing that TGFβ1 inhibits early progenitor proliferation and decreases the number of differentiation mitosis to accelerate terminal erythroid differentiation through different Smad2/3-Smad4 or -TIF-1γ signalings.5,6
Interestingly, the present work supports the hypothesis that terminal erythroid differentiation is mainly regulated by apoptosis. It has been hypothesized that erythroid precursors exhibit differential sensitivity toward EPO. In a steady state, low EPO levels support only the most EPO-sensitive progenitors for terminal differentiation, whereas the others undergo apoptosis. During erythropoietic stress, increased EPO levels induce a massive rapid terminal differentiation from the entire compartment of erythroid progenitors (see figure). Thus, TGFβ1 restrains this EPO-responsive erythroid compartment, preventing an EPO response in case of erythropoietic stress. This important role of TGFβ1 in the regulation of erythropoiesis is a bit surprising, as recent studies have underscored the role of other members of the TGFβ superfamily. Indeed, ActRIIA/B ligand traps are able to increase RBC production both in a steady stage and in several diseases, such as myelodysplasia or β-thalassemia.7 This effect is mediated through binding of GDF11 and probably other members of the activin/GDF family that are cleared, however, not by TGFβ1 that does not bind ActRIIA and B. These opposite results may suggest that TGFβ1 and Activin/GDF11 play different complementary roles in erythropoiesis.
This study raises several questions related to MK-derived TGFβ1:
TGFβ1 is stored in α-granules of MKs, and its secretion may be related to 2 mechanisms: either a leaky storage or a continuous α-granule content release without the activation process as required for platelets. It is not known if this process is cell autonomous or regulated by external sensors yet to be discovered. This will be important to investigate as deregulation of TGFβ1 secretion leads to myelofibrosis in myeloproliferative disorders or the gray platelet syndrome.8 At least, the present tgfb1ΔMk/ΔMk model could be useful to demonstrate the direct role of the MK-TGFβ1 in the genesis of myelofibrosis.
TGFβ1 is secreted by MKs as part of a latent complex bound to the extracellular matrix that needs to be cleaved to release its active form. This activation process is not yet fully understood but may involve integrin αvβ8 for TGFβ1-induced HSC dormancy by nonmyelinating Schwann cells.9 However, different mechanisms of activation may occur in the HSC niche and erythroid islands because exogenous TGFβ1, known to inhibit erythroid progenitors, could not rescue the erythroid phenotype in contrast to HSC quiescence.
Megakaryopoiesis and erythropoiesis have often been associated as they have a common bipotent progenitor, and excess production of one lineage often results in a default production of the other lineage. There is in vitro evidence that TGFβ1 regulates platelet production through a negative feedback mechanism. It would be interesting to study MK and platelet production in the tgfb1ΔMk/ΔMk mice. More broadly, MK-TGFβ1 blockade expands functional HSC and erythroid progenitors whose further differentiation toward high RBC production is blocked by the low EPO level. One can perceive that similar processes may occur for nonerythroid cells, such as granulocytes with granulocyte-colony-stimulating factor or platelets with thrombopoietin, both cytokines having this dual early-unspecific/late-specific action.
Altogether, this study provides new insights on the role of TGFβ1 in normal hematopoiesis and underscores the TGFβ1 signaling pathway as an important therapeutic target to correct HSC function and some cytopenia and anemia, as shown for myelodysplastic syndromes.10 It also emphasizes the important role of MK not only on HSC functions but also on the regulation of erythropoiesis.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal