In this issue of Blood, Zhu et al1 and Yvernogeau et al2 provide critical new information about the molecular and cellular mechanisms that drive the generation of hematopoietic stem cells (HSCs). Zhu et al reveal new stages in the development of HSCs and Yvernogeau et al identify and functionally validate novel regulatory molecules in the supporting microenvironment. These 2 new studies advance our understanding of this vital process and improve the prospect of producing clinical-grade HSCs in vitro.
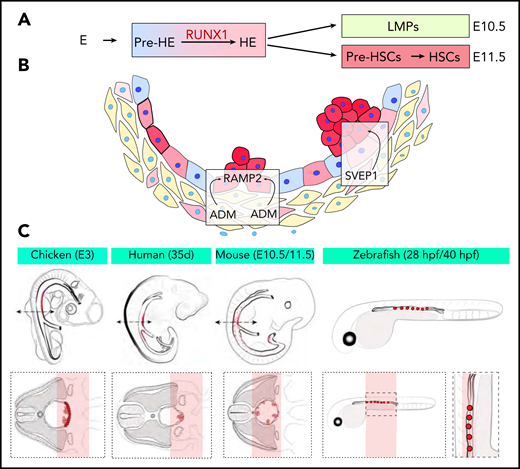
(A) New developmental trajectory from endothelium to pre-HSCs. An intermediate pre-HE stage generates HE cells through the upregulation of RUNX1. An initial wave of LMPs followed by a second wave of pre-HSCs contribute to hematopoietic clusters at E10.5 and E11.5, respectively. (B) The ligand-receptor couple ADM-RAMP2 and the secreted factor SVEP1 regulate HSPCs production in vivo. (C) Schemes of the embryo species and samples used for Tomo-seq. The red areas mark the ventral aortic region of emergence of clusters and pre-HSCs.
(A) New developmental trajectory from endothelium to pre-HSCs. An intermediate pre-HE stage generates HE cells through the upregulation of RUNX1. An initial wave of LMPs followed by a second wave of pre-HSCs contribute to hematopoietic clusters at E10.5 and E11.5, respectively. (B) The ligand-receptor couple ADM-RAMP2 and the secreted factor SVEP1 regulate HSPCs production in vivo. (C) Schemes of the embryo species and samples used for Tomo-seq. The red areas mark the ventral aortic region of emergence of clusters and pre-HSCs.
HSCs are widely used as a therapeutic option to help regenerate the hematopoietic system after treatment for leukemia or for gene therapy applications. Although bone marrow or mobilized peripheral blood cells are common sources of HSCs, the availability of an immune-compatible donor is often a limitation. Thus, the possibility of generating HSCs in vitro has been a central goal of researchers in the fields of hematopoietic and regenerative medicine for many years. Although proof-of-concept experiments have been performed, these processes are not yet robust enough to be clinical options. A better understanding of generating HSCs in vivo will improve our ability to generate these precious cells in vitro.
The vertebrate hematopoietic system is established by successive waves of blood cell generation.3 The first 2 waves take place in the extra-embryonic yolk sac and produce hematopoietic progenitors with restricted potential. The third and last wave takes place in the intra-embryonic aorta-gonad-mesonephros (AGM) region. This wave generates hematopoietic stem and progenitor cells (HSPCs), including the HSCs that will sustain the hematopoietic system during adulthood.4 Functional analysis of endothelial cells and lineage-tracing experiments performed in chicken, mice, and zebrafish have shown that all hematopoietic cells initially emerge from specialized endothelial cells. These hemogenic endothelium (HE) cells generate hematopoietic cells through a process called endothelial-to-hematopoietic-transition (EHT) (reviewed in Dzierzak and Bigas5 ). The transcription factor RUNX1 is critical for EHT.6,7 In its absence, HE cells do not lose their endothelial identity and remain mostly adherent; the few cells that detach from the endothelial lining apoptose. AGM hematopoiesis materializes with the formation of small groups of cells called hematopoietic clusters, which are found mainly on the ventral side of the aorta. This polarization of hematopoietic emergence suggests a critical role for the microenvironment, and in particular, the ventral mesenchyme of the AGM.8
In the article by Zhu et al, the authors performed state-of-the-art single-cell RNA-sequencing (scRNA-seq) and single-cell assay for transposase-accessible chromatin sequencing (scATAC-seq) on more than 37 000 murine cells undergoing EHT from embryonic day 9.5 to 11.5 (E9.5 to E11.5). After a developmental trajectory was reconstructed, they identified a new cellular state before HE, named pre-HE, in which the cells stall before undergoing EHT. They propose that the upregulation of RUNX1 expression induces the transition from pre-HE to HE and EHT and identified a candidate distal RUNX1 enhancer element potentially implicated in this increased RUNX1 expression. These findings confirm the previously reported exquisite sensitivity of EHT to RUNX1 levels9,10 and now identify 1 specific dependent step and a mechanism associated with the transition to full EHT. In addition, the authors also showed that hematopoietic clusters contain at least 2 types of progenitors: committed lympho-myeloid-biased progenitors (LMPs) at E10.5 and pre-HSCs at E11.5. This finding is relevant to current efforts to generate HSCs in vitro because lymphoid potential is often used as a surrogate for pre-HSC formation. These new data suggest that trying to define optimal conditions for the production of committed LMPs might not be the best option for improving pre-HSC production from embryonic stem cell cultures ex vivo.
In the article by Yvernogeau et al, the authors deploy another state-of-the-art technique, RNA tomography sequencing (Tomo-seq), to identify critical components within the ventral microenvironment that supports the generation of HSCs. In that approach, AGM sections were sliced, mainly along the dorso-ventral axis, and were processed for RNA-seq to generate transcriptional maps. Tomo-seq was impressively conducted on embryos of all of the 4 different species most commonly used to study developmental hematopoiesis: chicken, zebrafish, mouse, and human. Comparative data analyses identified a set of 3 genes (Podxl, Aldh1a2, and Ppargc1a) expressed in the aortic microenvironment in all 4 species. All of these genes were previously implicated in regulating hematopoiesis and probably represent an under-determination of the overlap between species because of incomplete detection of gene expression. To next explore receptor-ligand crosstalk between the microenvironment and HSCs, the authors combined spatial Tomo-seq maps with scRNA-seq data sets from clusters of mouse and chicken embryos. They identified and functionally validated the ligand-receptor couple known as adrenomedullin and adrenomedullin-receptor activity-modifying protein 2 (ADM-RAMP2) as a critically conserved ligand-receptor pair involved in HSPC production in vivo. They also identified the secreted factor SVEP1 as the first extrinsic factor identified in the aortic microenvironment that regulates cluster cellularity and fate in the mouse embryo.
Together, these 2 new technological tour de force articles provide our community with immediate new insights into the development of HSCs and also with a wealth of information in the interrogable data set resources associated with each study (https://github.com/qinzhu/VisCello.eht; http://multi-species.embryos.tomoseq.genomes.nl). We are now getting closer to the tantalizing prospect of generating complete spatial and temporal transcriptional maps of the aortic niche and of the cells becoming HSCs. One limitation of the current Tomo-seq approach is that gene expression is not entirely at the single-cell level. However, this can be resolved by additional integration with scRNA-seq or it might be addressed by new developments via spatial transcriptomics approaches. Similarly, we can expect new technical improvements in the sensitivity of single-cell transcriptomics as well as in the ability to probe the epigenetic landscape at a single-cell level. The ultimate challenge is to use these new findings to generate clinical-grade HSCs in vitro.
Conflict-of-interest disclosure: G.L. declares no competing financial interests.