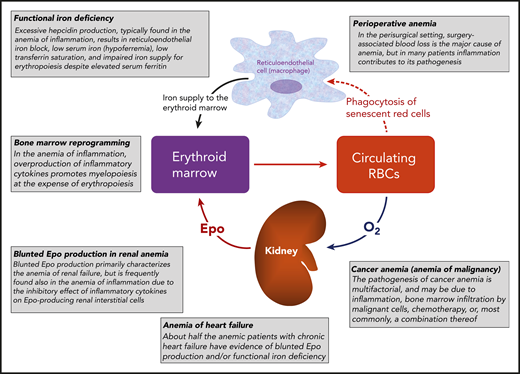
The human erythron has been defined by Hillman and Finch1 as the highly specialized tissue that is responsible for oxygen transport. Key components of the erythron are the erythroid marrow, which is responsible for red cell production (erythropoiesis), and circulating red blood cells, which transport oxygen from lungs to body tissues (Figure 1). Several factors control the differentiation and maturation of erythroid progenitors and precursors in the bone marrow,2 but a major role is played by erythropoietin (Epo). This glycoprotein is synthesized by interstitial fibroblastlike cells in the kidney, circulates in peripheral blood as a true hormone, and participates in a classic negative-feedback control system.3 In the broader sense, the human erythron includes the reticuloendothelial system, where red cells are phagocytized at the end of their life span, hemoglobin is catabolized, and iron is released into circulation to be transported by transferrin to body tissues, primarily to the erythroid marrow (Figure 1).
A representation of the human erythron and an illustration of common mechanisms of anemia. This figure is intended to help the reader better understand the treatment of disease-related and perisurgical anemias considered in the “How I Treat” series of this issue of Blood; that is: renal anemia, anemia in heart failure, anemia in cancer, and anemia in the perisurgical setting.
A representation of the human erythron and an illustration of common mechanisms of anemia. This figure is intended to help the reader better understand the treatment of disease-related and perisurgical anemias considered in the “How I Treat” series of this issue of Blood; that is: renal anemia, anemia in heart failure, anemia in cancer, and anemia in the perisurgical setting.
Several abnormalities of the human erythron can result in impaired red cell production and anemia. In chronic kidney disease, blunted Epo production represents a major factor in the pathogenesis of anemia, and administration of recombinant human Epo can increase the hemoglobin level in these patients.3 In the anemia caused by inflammation or chronic disease, the overproduction of inflammatory cytokines alters several biological pathways of the human erythron, leading to blunted Epo production, a defective iron supply for erythropoiesis, and promotion of myelopoiesis at the expense of erythropoiesis (Figure 1).4 The defective iron supply for erythropoiesis occurs in a condition characterized by increased hepcidin production, reticuloendothelial iron block, elevated serum ferritin, and low serum iron and transferrin saturation. This condition is commonly referred to as functional iron deficiency.5,6 Interestingly, approximately half of patients with anemia in chronic heart failure have evidence of blunted endogenous Epo production and/or functional iron deficiency.7 Anemia is common in patients with malignancy, and its pathogenesis is typically multifactorial.8 In untreated patients, the most common type is, again, induced by inflammation, whereas bone marrow infiltration by malignant cells is typically found in patients with hematologic malignancies. Chemotherapy may cause or worsen anemia in patients with cancer, and recombinant human Epo can be useful in its treatment.9 Surgery-associated blood loss is the major determinant of perioperative anemia, but many patients also have a component of inflammation-induced anemia.10
Figure 1 summarizes the main pathogenetic mechanisms of anemia in chronic kidney disease, heart failure, malignancy, and the perisurgical setting. The “How I Treat” series in this issue of Blood includes articles written by expert clinicians who offer guidance regarding the treatment of disease-related and perisurgical anemias:
Steven Fishbane and Daniel W. Coyne, “How I treat renal anemia”
Inder Anand and Pankaj Gupta, “How I treat anemia in heart failure”
Jeffrey A. Gilreath and George M. Rodgers, “How I treat cancer-associated anemia”
Aryeh Shander, Margit Kaufman, and Lawrence T. Goodnough, “How I treat anemia in the perisurgical setting”
I hope that these articles will help Blood readers improve their knowledge of the optimal treatment for anemia in these conditions.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal