In this issue of Blood, Wu and colleagues1 report that trimethylamine N-oxide (TMAO), an intestinal microbiome-dependent metabolite, worsens graft-versus-host disease (GVHD). They further found that TMAO induces M1 polarization of bone marrow–derived macrophages via the nucleotide-binding oligomerization domain–like receptor protein 3 (NLRP3). TMAO is produced by hepatic processing of intestinal bacteria–derived trimethylamine (TMA) following metabolism of certain dietary nutrients, including choline, lecithin, l-carnitine, and γ-butyrobetaine.
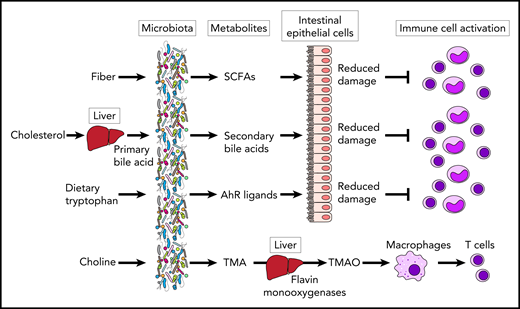
Microbial metabolites modulate immune cell responses in GVHD. SCFAs derived from fiber and secondary bile acids protect epithelial cells from damage, leading to suppression of immune cell activation. AhR ligands derived from dietary tryptophan can also suppress immune cell activation through reduction of intestinal epithelial damage in GVHD. TMAO is produced by hepatic processing by flavin monooxygenases of TMA following bacterial metabolism of choline and other dietary components. TMAO augmented alloreactive T-cell proliferation via M1 polarization of bone marrow–derived macrophages.
Microbial metabolites modulate immune cell responses in GVHD. SCFAs derived from fiber and secondary bile acids protect epithelial cells from damage, leading to suppression of immune cell activation. AhR ligands derived from dietary tryptophan can also suppress immune cell activation through reduction of intestinal epithelial damage in GVHD. TMAO is produced by hepatic processing by flavin monooxygenases of TMA following bacterial metabolism of choline and other dietary components. TMAO augmented alloreactive T-cell proliferation via M1 polarization of bone marrow–derived macrophages.
GVHD is an alloreactive, donor T-cell–mediated inflammatory disease that occurs often after allogeneic hematopoietic stem cell transplantation (allo-HSCT) and can involve the skin, liver, and gastrointestinal tract. The success of allo-HSCT continues to be significantly limited by GVHD, even though the field has seen improvements in both prevention and clinical management. Targeting intestinal bacteria may be a promising novel approach to further improve allo-HSCT clinical outcomes. A disrupted intestinal microbiome, also known as dysbiosis, occurs frequently in allo-HSCT recipients and can manifest as loss of obligately anaerobic commensal bacteria, expansion of pathogenic bacteria, and reduced microbiome diversity. Microbiome injury, in turn, has been strongly associated with acute GVHD and reduced overall survival.2 However, the mechanisms by which GVHD severity is modulated by intestinal bacteria are not fully understood. Recent studies have uncovered relationships between the microbiome and microbiome-derived metabolites in both mouse models and the clinical setting. Metabolites such as short-chain fatty acids (SCFAs) can support tissue repair and regulate immune cell activation.3,4 Bile acids have also been identified as potent regulators of the immune system through the inhibition of the NLRP3-dependent inflammasome pathway.5 Aryl hydrocarbon receptor ligands (AhR) such as indole-3-aldehyde can directly regulate innate immunity and modulate T helper (Th)17 responses and promote tolerance via regulatory T cells and type 1 regulatory cells.6 In a metabolomic analysis of patients with acute GVHD, Michonneau and colleagues7 recently demonstrated that acute GVHD is associated with alterations in bile acids and decreased production of AhR ligands by the microbiome (see figure).
Wu and colleagues focused on a particularly well studied microbiome-derived metabolite, TMAO, which has been associated with cardiovascular complications arising from atherosclerosis and thrombosis, but has not been known to affect GVHD. TMAO can induce vascular inflammation and endothelial dysfunction by formation and activation of NLRP3 inflammasomes in endothelial cells. The results show a novel link between TMAO and GVHD in mouse models and further demonstrate that TMAO induces secretion of M1-like cytokines from bone marrow–derived macrophages in an NLRP3-dependent fashion. TMAO augments alloreactive T-cell proliferation and Th1 subtype differentiation in mice with GVHD but not in an in vitro T-cell culture system, leading to an investigation to determine the role of TMAO in macrophage polarization.
NLRP3 has been shown to have conflicting contributions to GVHD. NLRP3-mediated signaling is essential for intestinal commensal bacterial products and the damage-associated molecular pattern that leads to interleukin-1 production.8 SCFAs, however, can also activate the NLRP3 inflammasome, and signaling in host nonhematopoietic cells has been found to be critical for reducing the severity of GVHD.4 The seemingly opposing effects of NLRP3 on GVHD may be related to differential activation in distinct host tissue compartments or immune cell populations, and further investigation is needed to better clarify the effects of microbiome-derived products in NLRP3-modulated GVHD.
High plasma levels of TMAO are associated with a variety of adverse outcomes, including cardiovascular diseases, glucose intolerance, kidney damage, obesity, and diabetes, although other clinical factors, such as age, race, and cholic acid and bile salt levels, have also been associated. In patient who undergo allo-HSCT, plasma TMAO levels could be a novel biomarker for predicting GVHD, and an evaluation is warranted, given the preclinical findings by Wu and colleagues. One question is how TMAO levels are affected by anorexia and other dietary alterations, as well as antibiotic use, all of which are common in the setting of allo-HSCT.
If a clinical association is found, how could we target TMAO in allo-HSCT patients? Diet is known to play a key role in TMAO formation. TMAO can be found in fish or may be secondarily produced by bacterial metabolism of red meat or eggs, which are rich in choline and l-carnitine. Thus, strategies to reduce TMAO include limiting dietary intake of animal fat and protein, consuming a vegetarian diet, use of prebiotics/probiotics, and even supplementation with pistachios.9 Identification of bacterial subsets responsible for increased TMAO is another approach. The Firmicutes/Bacteroidetes ratio has been used to predict TMAO concentrations in plasma. Certain specific bacteria have also been identified as important in TMAO production, including Anaerococcus hydrongenalis, Clostridium asparagiforme, Clostridium hathewayi, Clostridium sporogenes, Edwardsiella tarda, Escherichia fergusonii, Proteus penneri, and Providencia rettgeri.10 Targeting those may be a challenging strategy, however, because they are taxonomically diverse and have not been reported to be associated with increased GVHD. Broad-spectrum antibiotics can almost totally suppress the production of TMAO by eliminating the gut microbiome. However, the levels of TMAO return to normal after withdrawal of antibiotics, and the long-term use of broad-spectrum antibiotics is not feasible given the likely selection for resistant bacteria. In the current study, the Wu et al demonstrated that administration of 3,3-dimethyldimethyl-1-butanol, an inhibitor of microbial TMA lyases, is effective in ameliorating aggravated GVHD. Blockade of hepatic flavin monooxygenases, which convert TMA to TMAO, is also being investigated in a clinical trial (www.clinicaltrials.gov #NCT03152097).
In summary, the findings in this study suggest that the microbial metabolite TMAO can be a potent contributor to the severity of acute GVHD. Further studies in both animal models and human biospecimens could clarify the importance of the role played by TMAO with respect to the dynamics of the microbiome and microbial metabolites and the modulation of the immune system in the development of acute GVHD.
Conflict-of-interest disclosure: R.R.J. has consulted for Karius, Merck, Microbiome DX, and Prolacta; is on the scientific advisory boards of Kaleido, Maat Pharma, and Seres; and has received patent royalties licensed to Seres. E.H. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal