Key Points
Brain SWI demonstrates occult cerebral microbleeds in almost 50% of patients with ITP and platelet counts less than 30 × 109/L.
CMBs are associated with longer disease duration and lower nadir platelet count and may be a useful noninvasive marker of hemorrhagic risk.
Abstract
Management of symptoms and prevention of life-threatening hemorrhage in immune thrombocytopenia (ITP) must be balanced against adverse effects of therapies. Because current treatment guidelines based on platelet count are confounded by variable bleeding phenotypes, there is a need to identify new objective markers of disease severity for treatment stratification. In this cross-sectional prospective study of 49 patients with ITP and nadir platelet counts <30 × 109/L and 18 aged-matched healthy controls, we used susceptibility-weighted magnetic resonance imaging to detect cerebral microbleeds (CMBs) as a marker of occult hemorrhage. CMBs were detected using a semiautomated method and correlated with clinical metadata using multivariate regression analysis. No CMBs were detected in health controls. In contrast, lobar CMBs were identified in 43% (21 of 49) of patients with ITP; prevalence increased with decreasing nadir platelet count (0/4, ≥15 × 109/L; 2/9, 10-14 × 109/L; 4/11, 5-9 × 109/L; 15/25 <5 × 109/L) and was associated with longer disease duration (P = 7 × 10−6), lower nadir platelet count (P = .005), lower platelet count at time of neuroimaging (P = .029), and higher organ bleeding scores (P = .028). Mucosal and skin bleeding scores, number of previous treatments, age, and sex were not associated with CMBs. Occult cerebral microhemorrhage is common in patients with moderate to severe ITP. Strong associations with ITP duration may reflect CMB accrual over time or more refractory disease. Further longitudinal studies in children and adults will allow greater understanding of the natural history and clinical and prognostic significance of CMBs.
Introduction
Immune thrombocytopenia (ITP) is an uncommon autoimmune disorder in which an aberrant immune response results in thrombocytopenia, variable bleeding symptoms, fatigue, and impaired health-related quality of life.1 A number of treatments are effective at increasing the platelet count, including steroids, other immunosuppressants, rituximab, splenectomy, and targeted thrombopoietin receptor agonists. Unfortunately, few are curative, and adults with ITP have a remission rate with medical therapy of only 30%.
Management strategies are therefore either symptom-directed or aimed to prevent more serious bleeding. However, as identified in the recent American Society of Hematology guidelines for ITP, there are limited data to guide management decisions.2 Intracranial hemorrhage (ICH) is the most serious bleeding complication in patients with ITP and is associated with substantial morbidity and mortality,3 yet existing methods to identify those patients at risk of serious bleeding have limited value. Although hematuria has been associated with development of ICH,3 skin and mucosal bleeding scores do not have predictive value.4-6 Consequently, the platelet count is frequently used to inform decisions to treat. Current guidelines recommend that adults with a platelet count <20 to 30 × 109/L receive treatment, irrespective of bleeding symptoms.1,2,7 Patients with the same platelet count, however, commonly exhibit very different degrees of bleeding symptoms. In contrast, children are currently treated according to bleeding symptoms, regardless of platelet count; this approach is partly informed by the low likelihood of severe bleeding and high spontaneous remission rate (80%-90%) in children.7 The incidence of occult or subclinical hemorrhage as a consequence of ongoing thrombocytopenia in ITP has not been systematically explored. Detection of such occult bleeding could identify hemorrhagic tendency in individual patients and better inform treatment strategies.
Cerebral microbleeds (CMBs) or microhemorrhages are tiny hemosiderin deposits, which can be detected in the brain noninvasively using susceptibility-sensitive magnetic resonance imaging (MRI) techniques.8 In older populations, the presence of CMBs is associated with increased rates of dementia and vascular events.9,10 CMBs are also characteristically identified and associated with cognitive impairment in specific disease conditions; for example, cerebral amyloid angiopathy11 and traumatic diffuse axonal brain injury12 and as a long-term effect of cranial radiotherapy.13,14 Only 1 previous published study has explored CMBs in ITP, where a CMB was identified on gradient echo T2* MRI in 1 of 27 children with short duration of ITP.15 The overall frequency of CMBs, their relationship with severity and duration of ITP, and clinical bleeding scores are, however, unknown.
Although CMBs are detectable using widely used gradient echo T2* MRI sequences, sensitivity to the presence of blood products is further improved in more recently available susceptibility-weighted imaging (SWI), which exploits phase information from the MRI signal to generate additional tissue contrast.16 We hypothesized that imaging the brain for CMBs with SWI could provide a sensitive noninvasive biomarker of occult hemorrhage in ITP; this will identify patients with more hemorrhagic phenotypes and may as a result improve future stratification of treatment. This prospective study was designed to explore the incidence of CMBs in patients with ITP using SWI of the brain and understand the relationship between CMBs and platelet count, bleeding scores, disease duration, and treatment history.
Methods
This study was approved by London-Surrey Borders Health Research Authority Ethics Committee and was conducted in accordance with the Declaration of Helsinki. Fifty patients attending the Hammersmith Hospital’s ITP Center (London, United Kingdom) were prospectively recruited between 2014 and 2018. Recruitment criteria included a diagnosis of ITP defined according to the International Working Party7 as a platelet count <100 × 109/L with exclusion of other causes of thrombocytopenia. Patients had at least 1 count <30 × 109/L during the course of their disease and no contraindications to MRI (eg, severe claustrophobia, metallic implants, shunts).
A dedicated imaging protocol including SWI (repetition time/echo time = 28 ms/20 ms, flip angle = 15, 0.5- × 0.5-mm in-plane resolution, 1-mm slice thickness, 35- × 45-cm FOV) was acquired on a 3-T clinical MRI system (VB19; Siemens Verio, Erlangen, Germany) for patients and 18 age-matched healthy controls. Although the same imaging protocol was used for both cohorts, the healthy controls were recruited separate under a different ethics approval. CMBs were detected with a user-guided, semiautomated intensity-based detection algorithm17,18 and verified by an experienced neuroradiologist blinded to clinical details. Skin mucosal organ (SMOG) bleeding scores were graded from 0 (best) to 5 (worst).6 In addition to imaging data and bleeding score, clinical data including patient age, sex, current and lowest recorded platelet count (nadir), duration of disease, and treatment history were collected.
A series of univariate Poisson regressions were performed to explore potential relationships between the total number of CMBs on SWI and clinical data. SMOG bleeding scores were factored into 2 groups: a bleeding score of 0 or 1 or a bleeding score of 2 or greater. Variables significantly associated with presence of CMBs in univariate analysis were included in a multivariate model. Patient age and sex were modeled as covariates.
Results
Patient demographics are summarized in Table 1. One outlier with more than 50 CMBs had previously received total body irradiation for a stem cell transplantation and was therefore excluded from further analysis. SMOG bleeding scores were available for 42 of the remaining 49 patients. The healthy control group included 18 subjects (6 female) ranging from 21 to 64 years (mean, 45.4 years).
Patient demographics
| Clinical/demographic characteristics . | Patients without CMBs (N = 28) . | Patients with CMBs (N = 21) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Female, no. of patients (%) | 15 (54) | 14 (67) | ||||||
| Age at time of MRI (y), mean (range) | 42 (20-84) | 40 (18-69) | ||||||
| Age at time of diagnosis (y), mean (range) | 37 (15-83) | 29 (3-63) | ||||||
| Disease duration (mo), mean (range) | 63 (1-232) | 124 (4-486) | ||||||
| Platelet count day of MRI, mean (range) | 109 (11-393) | 73 (4-327) | ||||||
| Lowest platelet count, mean (range) | 8 (0-25) | 4 (1-13) | ||||||
| Number of prior treatments, mean (range) | 4 (1-8) | 6 (1-17) | ||||||
| Bleeding score | 0-1 | 2 | 3 | 4-5 | 0-1 | 2 | 3 | 4-5 |
| Skin, no. of patients | 6 | 7 | 11 | 0 | 6 | 4 | 7 | 0 |
| Visible mucosae, no. of patients | 14 | 8 | 2 | 0 | 8 | 5 | 4 | 0 |
| Organs, no. of patients | 15 | 6 | 3 | 0 | 10 | 1 | 5 | 1 |
| Diabetes, no. of patients | 0 | 0 | ||||||
| Hypertension, no. of patients | 1 | 1 | ||||||
| Heart disease, no. of patients | 1 | 2 | ||||||
| Antiplatelet/anticoagulant agents, no. of patients | 2 | 2 | ||||||
| Clinical/demographic characteristics . | Patients without CMBs (N = 28) . | Patients with CMBs (N = 21) . | ||||||
|---|---|---|---|---|---|---|---|---|
| Female, no. of patients (%) | 15 (54) | 14 (67) | ||||||
| Age at time of MRI (y), mean (range) | 42 (20-84) | 40 (18-69) | ||||||
| Age at time of diagnosis (y), mean (range) | 37 (15-83) | 29 (3-63) | ||||||
| Disease duration (mo), mean (range) | 63 (1-232) | 124 (4-486) | ||||||
| Platelet count day of MRI, mean (range) | 109 (11-393) | 73 (4-327) | ||||||
| Lowest platelet count, mean (range) | 8 (0-25) | 4 (1-13) | ||||||
| Number of prior treatments, mean (range) | 4 (1-8) | 6 (1-17) | ||||||
| Bleeding score | 0-1 | 2 | 3 | 4-5 | 0-1 | 2 | 3 | 4-5 |
| Skin, no. of patients | 6 | 7 | 11 | 0 | 6 | 4 | 7 | 0 |
| Visible mucosae, no. of patients | 14 | 8 | 2 | 0 | 8 | 5 | 4 | 0 |
| Organs, no. of patients | 15 | 6 | 3 | 0 | 10 | 1 | 5 | 1 |
| Diabetes, no. of patients | 0 | 0 | ||||||
| Hypertension, no. of patients | 1 | 1 | ||||||
| Heart disease, no. of patients | 1 | 2 | ||||||
| Antiplatelet/anticoagulant agents, no. of patients | 2 | 2 | ||||||
Presence of CMBs
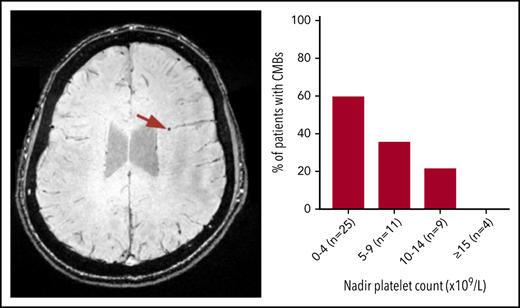
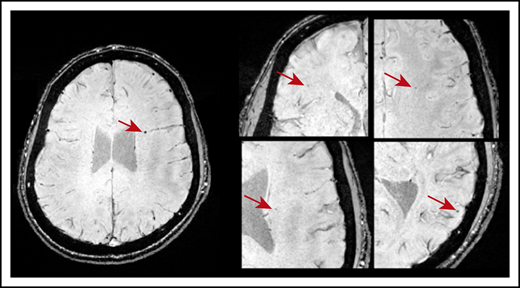
CMBs were identified in 21 of 49 patients with ITP (43%). Most CMBs were between 1 and 5 mm in diameter and variably distributed throughout the brain (Figure 1). Six patients (12%) had 1 CMB; 11 (22%) had between 2 and 4 CMBs; and 5 patients (9.8%) exhibited 5 or more CMBs. Fourteen of 33 patients younger than 45 years had CMBs: 5 had 1 CMB and 9 patients had >1 CMB. Three of 8 patients in the age range of 45 to 60 years had CMBs; 1 had 1 CMB and 2 had >1 CMB. Four of 8 patients older than 60 years had >1 CMB. No CMBs were identified in any of the 18 healthy control subjects.
Axial SWI showing CMBs (indicated by the red arrows) in a patient who was diagnosed with ITP at age 10. This patient was 20 years old at the time of MRI and had a disease duration of 118 months and 5 CMBs dispersed throughout the brain.
Axial SWI showing CMBs (indicated by the red arrows) in a patient who was diagnosed with ITP at age 10. This patient was 20 years old at the time of MRI and had a disease duration of 118 months and 5 CMBs dispersed throughout the brain.
Factors associated with CMBs
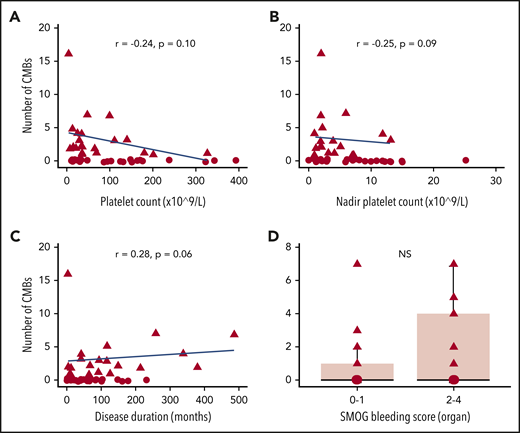
Multivariate analysis revealed that CMBs were more likely in patients with lower platelet counts, both nadir (P = .005, α = .01) and at time of MRI (P = .029, α = .05), with increasing disease duration (P = 7.11 × 10−6, α = .001) and higher SMOG organ bleeding scores (P = .028, α = .05) when adjusted for age and biological sex (Figure 2). Skin SMOG score were not associated with CMBs, and mucosal SMOG scores showed association only in the univariate analysis. P values and incidence rate ratios for the univariate and multivariate analyses are listed in Table 2.
Visual representation of the relationship between number of CMBs and significant risk factors. (A) Platelet count at time of MRI. (B) Nadir platelet count (lowest recorded). (C) Disease duration. (D) Organ bleeding score. Each data point corresponds to a different patient with ITP. For better visualization, patients without CMBs were labeled with a different shape, the outlier patient with 56 bleeds was excluded, and the range of panel D was set to a maximum of 8 (not all patients shown in panels A-C had organ bleeding scores).
Visual representation of the relationship between number of CMBs and significant risk factors. (A) Platelet count at time of MRI. (B) Nadir platelet count (lowest recorded). (C) Disease duration. (D) Organ bleeding score. Each data point corresponds to a different patient with ITP. For better visualization, patients without CMBs were labeled with a different shape, the outlier patient with 56 bleeds was excluded, and the range of panel D was set to a maximum of 8 (not all patients shown in panels A-C had organ bleeding scores).
Multivariate analysis of CMB presence and clinical characteristics of patients with ITP
| Characteristic . | Univariate Poisson analysis . | Multivariate Poisson analysis . | ||
|---|---|---|---|---|
| Incidence rate ratio . | P . | Incidence rate ratio . | P . | |
| Disease duration, each additional month | 1.00 (1.00, 1.01) | 2.06 × 10−5*** | 1.01 (1.00, 1.01) | 7.11 × 10−6*** |
| Platelet count, at time of MRI | 0.992 (0.988, 0.996) | 2.71 × 10−4*** | 0.994 (0.989, 1.00) | .029* |
| Platelet count, low point | 0.879 (0.824, 0.939) | 1.28 × 10−4*** | 0.867 (0.786, 0.957) | .005** |
| Number of treatments, each additional treatment | 1.10 (1.03, 1.16) | .003** | 0.928 (0.831, 1.04) | .187 |
| SMOG (≥2), organ† | 3.27 (1.86, 5.76) | 4.10 × 10−5*** | 2.32 (1.10, 4.90) | .028* |
| SMOG (≥2), mucosal† | 2.40 (1.29, 4.48) | .006** | 1.09 (0.444, 2.65) | .857 |
| SMOG (≥2), skin† | 0.837 (0.469, 1.49) | .547 | Not included | — |
| Age, each additional year | 0.995 (0.980, 1.01) | .534 | 1.01 (0.990, 1.03) | .350 |
| Sex, male | 1.63 (1.02, 2.60) | .042* | 0.786 (0.382, 1.62) | .513 |
| Characteristic . | Univariate Poisson analysis . | Multivariate Poisson analysis . | ||
|---|---|---|---|---|
| Incidence rate ratio . | P . | Incidence rate ratio . | P . | |
| Disease duration, each additional month | 1.00 (1.00, 1.01) | 2.06 × 10−5*** | 1.01 (1.00, 1.01) | 7.11 × 10−6*** |
| Platelet count, at time of MRI | 0.992 (0.988, 0.996) | 2.71 × 10−4*** | 0.994 (0.989, 1.00) | .029* |
| Platelet count, low point | 0.879 (0.824, 0.939) | 1.28 × 10−4*** | 0.867 (0.786, 0.957) | .005** |
| Number of treatments, each additional treatment | 1.10 (1.03, 1.16) | .003** | 0.928 (0.831, 1.04) | .187 |
| SMOG (≥2), organ† | 3.27 (1.86, 5.76) | 4.10 × 10−5*** | 2.32 (1.10, 4.90) | .028* |
| SMOG (≥2), mucosal† | 2.40 (1.29, 4.48) | .006** | 1.09 (0.444, 2.65) | .857 |
| SMOG (≥2), skin† | 0.837 (0.469, 1.49) | .547 | Not included | — |
| Age, each additional year | 0.995 (0.980, 1.01) | .534 | 1.01 (0.990, 1.03) | .350 |
| Sex, male | 1.63 (1.02, 2.60) | .042* | 0.786 (0.382, 1.62) | .513 |
Significant at an α level of .001.
Significant at an α level of .01.
Significant at an α level of .05.
Bleeding score were factored into 2 groups: a score of 0 or 1 or a score of 2 or greater. Scores were not available for 8 patients.
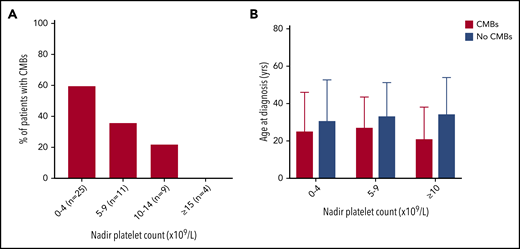
The proportion of patients with CMBs increased with lower nadir platelet count (Figure 3). CMBs were identified in 15 of 25 (60%) patients with nadir counts less than 5 × 109/L, 4 of 11 (36%) patients with nadir counts between 5 and 9 × 109/L, and 2 of 9 (22%) patients with counts between 10 and 14 × 109/L, whereas patients with lowest counts above 15 × 109/L did not have CMBs (n = 4). This observation was not influenced by age at presentation or duration of ITP.
The proportion of patients with CMBs increased with lower nadir platelet count. (A) With decreasing nadir platelet count, there was a greater percentage of patients with CMBs. There were no differences in age at diagnosis and only subtle differences in disease duration between these groups. (B) For patients in a given nadir platelet count range, age at diagnosis did not significantly affect CMB incidence.
The proportion of patients with CMBs increased with lower nadir platelet count. (A) With decreasing nadir platelet count, there was a greater percentage of patients with CMBs. There were no differences in age at diagnosis and only subtle differences in disease duration between these groups. (B) For patients in a given nadir platelet count range, age at diagnosis did not significantly affect CMB incidence.
Nine patients with a median nadir platelet counts of 2 (range, 1-12) had been diagnosed with ITP in childhood (median age, 14 years; range, 3-17 years), with a median disease duration of 118 months (range, 41-338 months) at the time of the MRI. Six of these 9 patients had CMBs, 3 of whom had been managed with observation and received only rescue treatment of severe bleeding events over a period of more than 10 years. Patients with more than 5 CMBs included 3 patients refractory to multiple lines of treatment: 1 who had also suffered a minor nonconcussive head injury when the platelet count was 1 × 109/L and 1 patient with a long duration of thrombocytopenia with no treatment (diagnosed in childhood, platelet count between 5 and 15 × 109/L for >10 years).
Discussion
Using SWI, we showed for the first time that nearly half of patients with nadir platelet count less than 15 × 109/L have CMBs in varying lobar regions of the brain. CMBs were not identified in any of the healthy control subjects in this study. This is consistent with historical data where CMBs are not observed in healthy young individuals,10,19 and their detection in patients younger than 45 years can therefore be attributed confidently to ITP. Detection of CMBs is known to increase with age in healthy individuals, although the rate of 44% (7 of 16) in patients 45 years or older in our cohort is still much higher than the prevalence of 6.5% at age 45 to 50 years, rising to 16.8% at age 60 to 69 years reported by the largest population-based study of CMBs in healthy aging individuals.20 The substantially higher CMB incidence in our study compared with the only previously published work in children with ITP is likely to reflect longer disease duration, differing adult and pediatric disease phenotypes, and greater sensitivity of SWI compared with gradient echo T2* sequences.
Our results demonstrate a clear link between severe thrombocytopenia and occult brain microhemorrhage, evidenced by increasing incidence of CMBs with lower platelet counts, both at nadir and at the time of MRI. More than 50% of patients with nadir platelet counts less than 15 × 109/L, however, did not have detectable CMBs, despite similar average age and disease duration. Platelet count alone is therefore an important but incomplete predictor of occult hemorrhage in patients with ITP. This variation in presence of CMBs at the same platelet count, and the lack of association with skin and mucosal bleeding scores suggests that SWI provides a specific noninvasive biomarker of central nervous system hemorrhagic tendency that could enable further stratification of disease phenotypes.
The strong association observed with disease duration could indicate that CMBs accumulate over time but may also reflect a group of patients with more refractory disease. This agrees with other data showing poorer clinical outcome in patients with long durations of thrombocytopenia.21
Although the study was not designed to predict ICH risk, a significant association was noted between CMB and high organ bleeding scores, and the 1 patient in our study who had previously had ICH also had one of the highest CMB counts. These observations are in keeping with the hypothesis that CMBs reflect risk of more significant bleeding.
Existing cohort studies have shown a correlation between platelet count less than 20 × 109/L and bleeding,22 and current clinical guidelines recommend a platelet threshold of 20 to 30 × 109/L for treatment in adults.7 No CMBs were detected in patients with nadir platelet counts >15 × 109, although the very small number in this group limits the conclusions that can be drawn about their absence in those with higher counts.
A number of patients in this cohort were diagnosed with ITP in childhood and some were not treated for long periods of time, in accordance with current pediatric guidelines. The high incidence of CMBs, including multiple lesions, in this subgroup and the lack of correlation between CMB and skin or mucosal bleeding scores suggests that current approaches to treatment based on clinical symptomatology alone may not appropriately protect patients from occult CNS bleeds.
The cross-sectional nature of the imaging data limits insights into the natural history of microbleeds in ITP and, in particular, separating the effects of disease duration alone from refractory phenotypes. Furthermore, the absence of correlative data with cognition or fatigue also precludes evaluation of any associative or mechanistic links with CMBs; the clinical relevance of CMBs in ITP with regard to short- and long-term neurologic function is therefore not yet clear. CMBs are strongly associated with neurologic sequelae in a number of neurodegenerative conditions,8 after external beam radiotherapy13 and traumatic brain injury,12 and further, weaker associations with cognitive impairment are seen more widely in elderly populations.9 The pathophysiology underlying microhemorrhage in those circumstances is primarily of damage to brain microvasculature, whereas the mechanism by which CMBs develop in ITP is not yet clear.
Many patients with ITP have no symptoms, and deciding whether to treat can be difficult. Conversely, some patients are refractory to multiple treatments, and balancing the adverse effects of higher-intensity treatment against the potential risk of bleeding is challenging. Our findings indicate that applying a treatment threshold based on platelet count alone could result in some patients being overtreated and others undertreated.
Further longitudinal study of CMB in wider populations of children and adults with ITP and correlation with neurocognitive and fatigue features will allow greater understanding of their natural history and clinical and prognostic significance. Future SWI examination of patients who have had ICH will also help to establish whether CMB burden is indeed a risk factor for ICH.
In summary, brain SWI demonstrates that occult microhemorrhage is common in moderate to severe ITP and provides a noninvasive biomarker of bleeding tendency. Although the clinical and prognostic implications of these findings are not yet clear and we would therefore not recommend using brain SWI to inform current patient management, it may provide a useful future adjunct to existing criteria for clinical decision making in adults and children with this challenging disease.
For original data, please contact n.cooper@imperial.ac.uk.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was funded, in part, by the Imperial College National Institute for Health Research Bioresources Cancer Theme and Imaging Theme and the UK ITP Patient Support Organization.
Authorship
Contribution: N.C. and A.D.W. designed the study, analyzed the results, and wrote the paper; M.A.M. analyzed the MRI data and wrote the paper; J.B.B. analyzed the results and metadata and wrote the paper; and C.V., A.C.J.H., T.Y., D.P., A.M., M.A., A.L., and D.J.S. collected clinical metadata, consented patients, arranged MRI examinations, contributed to data analysis, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Nichola Cooper, 4S10C Commonwealth Bldg, Imperial College London Hammersmith Campus, White City, London Q12 0NN, United Kingdom; e-mail: n.cooper@imperial.ac.uk.
REFERENCES
Author notes
N.C. and M.A.M. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal