In this issue of Blood, Li et al1 have demonstrated that NCOA4, the autophagic receptor for ferritin, is necessary for the mobilization of liver iron stores during stress erythropoiesis in mice.
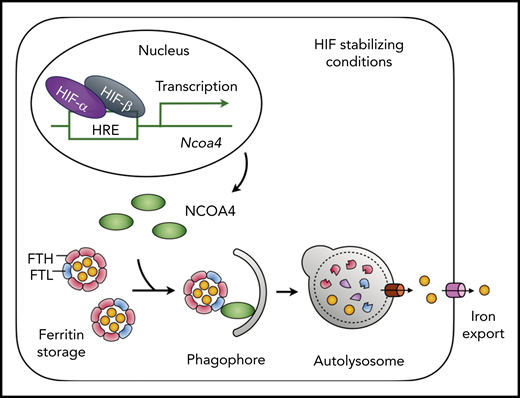
NCOA4 mediates iron mobilization from liver ferritin stores. HIF-1α and HIF-2α are stabilized under conditions that inhibit the activity of the prolyl hydroxylases that regulate HIF (eg, iron or oxygen deficiency, presence of small molecule inhibitors). HIF-driven transcription promotes expression of NCOA4, which is stable under conditions of iron deficiency. NCOA4 directs ferritin to the autolysosome for degradation, and iron released from ferritin is transferred to the cytosol for export via ferroportin. See Figure 7D in the article by Li et al that begins on page 2691.
NCOA4 mediates iron mobilization from liver ferritin stores. HIF-1α and HIF-2α are stabilized under conditions that inhibit the activity of the prolyl hydroxylases that regulate HIF (eg, iron or oxygen deficiency, presence of small molecule inhibitors). HIF-driven transcription promotes expression of NCOA4, which is stable under conditions of iron deficiency. NCOA4 directs ferritin to the autolysosome for degradation, and iron released from ferritin is transferred to the cytosol for export via ferroportin. See Figure 7D in the article by Li et al that begins on page 2691.
Acute blood loss, whether through hemorrhage, hemolysis, or phlebotomy, triggers erythropoiesis. Expansion of developing erythroid precursors necessitates mobilization of stored iron to accommodate increased synthesis of heme in the erythron. Where does all this iron come from? The short answer is ferritin. Although dietary iron absorption is increased during stress erythropoiesis, the most rapidly available source of iron is in this ubiquitous iron storage protein. Iron deposition into ferritin and mobilization out of ferritin are tightly controlled in mammals, and multiple modes of regulation affect both the “ins” and the “outs.” In 2014, nuclear receptor coactivator 4 (NCOA4) was identified as an autophagic receptor for ferritin, with a capacity to direct ferritin into autophagosomes destined for destruction in the lysosome.2,3 Here, Li and others show that NCOA4 is required to mobilize the iron stored in hepatocyte ferritin so that it may be used for stress erythropoiesis. Their work also suggests a new mode of iron-dependent transcriptional regulation for NCOA4 that involves the hypoxia-inducible factors 1 and 2.
In mammals, cytosolic ferritin is a symmetrical oligomeric complex, composed of 24 subunits of H- and l-chains, that form a hollow sphere into which iron is deposited.4 In cells, the amount of iron deposited into ferritin is largely proportional to the level of ferritin protein synthesized in the cytosol. The major means of controlling synthesis is through translational repression via the iron-regulatory proteins 1 and 2.5 When cells are iron depleted, the iron-regulatory proteins accumulate in their RNA-binding form, bind to the 5′ end of ferritin transcripts, and block translation of the ferritin messenger RNA (mRNA). Under conditions of iron excess, these same regulatory proteins lose their RNA-binding activity and ferritin mRNAs are actively translated. Thus, cells can expand their iron storage in ferritin when intracellular iron is abundant.
Cells also express cytosolic iron-binding proteins, termed iron chaperones, that deliver iron to ferritin through an iron-mediated protein–protein interaction.6 The major iron chaperone in mammalian cells is poly(rC)-binding protein 1 (PCBP1). PCBP1 is a multifunctional adapter protein that can bind both iron–glutathione complexes7 and cytosine-rich nucleic acids at separate sites on the protein. In developing erythroid cells, PCBP1 promotes α- and β-globin transcript stability through its RNA-binding activity. It also facilitates efficient iron delivery to ferritin through its iron chaperone activity. In both in vitro and in vivo models of red blood cell development in mice, transient, PCBP1-mediated, storage of iron in ferritin was necessary for efficient heme and hemoglobin synthesis.8
Iron is released from ferritin primarily through its degradation in the lysosome. This process occurs continuously at basal levels under conditions of iron balance and is upregulated when cells become iron starved.4 In cultured cells, ferritin degradation is controlled through the activity of NCOA4. NCOA4 binds to ferritin H-chain and recruits the complex to autophagic membranes. These membranes form autophagosomes that fuse with lysosomal membranes and release their contents into the lysosome for degradation. Lysosomal iron released from ferritin can be transported into the cytosol or mitochondria for use in iron cofactor synthesis or be exported from the cell via ferroportin, the sole iron efflux pump in mammals.
The major tissue for iron storage in mammals is the liver, where hepatocytes accumulate iron within ferritin. In order for ferritin iron within the liver to be used for erythropoiesis, the iron must be mobilized to leave the liver. Li and colleagues have addressed the question of whether NCOA4 in hepatocytes is needed to mobilize liver iron stores for use in erythropoiesis.1 Using hepatocyte-specific depletion of NCOA4 in mice, the authors induced acute iron deficiency and stress erythropoiesis using a combination of phlebotomy to produce anemia and dietary iron restriction to force mobilization of stored iron. Under these conditions, wild-type mice rapidly degraded ferritin and depleted non-heme iron stores in the liver. However, mice depleted of NCOA4 in hepatocytes were not able to degrade ferritin or mobilize liver iron stores. These mice exhibited evidence of cellular iron deficiency in the liver, with elevated expression of the transferrin receptor, despite the retention of ferritin iron stores. Thus, the NCOA4-mediated turnover of ferritin in hepatocytes is necessary for the mobilization of liver iron stores during acute iron deficiency (see figure).
In cells, NCOA4 levels are regulated by ubiquitin-mediated degradation in response to elevated cellular iron.9,10 Purified NCOA4 can directly bind iron in vitro. Elevated iron levels in cells lead to the recognition of iron-bound NCOA4 by the ubiquitin ligase HERC2, which results in the degradation of NCOA4 and the retention of ferritin in the cytosol. When iron levels are low, NCOA4 is not targeted by HERC2, and ferritin turnover is stimulated. However, Li and colleagues found that transcriptional mechanisms of NCOA4 regulation may also be at work in cultured human cells of hepatocyte origin.1 They observed that cell treatments that increased the activity of hypoxia-inducible factors (HIF) 1α or 2α increased mRNA levels of NCOA4. This transcriptional activation was lost in cells simultaneously depleted of HIF-1α and HIF-2α. Furthermore, they identified a canonical HIF-response element in control regions upstream of the NCOA4 locus.
Whether NCOA4 is regulated by HIF-1α or HIF-2α in murine liver and whether physiologic iron or oxygen depletion contribute to the transcriptional regulation of NCOA4 in mice was not addressed in these studies. Given the many layers of ferritin regulation that have been uncovered to date, further regulatory mechanisms involving NCOA4 seem likely. Modulating the turnover of ferritin could prove helpful in clinical situations. For example, enhanced ferritin turnover could facilitate the removal of excess iron during chelation therapy for disorders with iron overload, such as thalassemia or hereditary hemochromatosis.
Conflict-of-interest disclosure: The author declares no competing financial interests.