For decades, the expansion of hematopoietic stem cells (HSCs) has been an elusive goal. In this issue of Blood, Hua et al took a step toward reaching this goal by demonstrating that the addition of the small molecule bromodomain and extra-terminal motif (BET) inhibitor CPI203 to a cytokine cocktail results in expansion of serially transplantable long-term repopulating HSCs (LT-HSCs). They also showed enhancement of their potential to differentiate into megakaryocytes (MKs).1
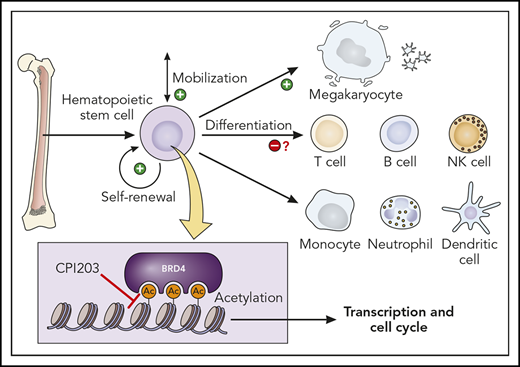
Effect of BET inhibition by CPI203 on hematopoiesis. Inhibiting BET proteins that bind acetylated lysine residues affects transcriptional regulation and the cell cycle. In HSCs, this results in increased self-renewal and expansion, mobilization, and differentiation into MKs. NK, natural killer. Professional illustration by Patrick Lane, ScEYEnce Studios.
Effect of BET inhibition by CPI203 on hematopoiesis. Inhibiting BET proteins that bind acetylated lysine residues affects transcriptional regulation and the cell cycle. In HSCs, this results in increased self-renewal and expansion, mobilization, and differentiation into MKs. NK, natural killer. Professional illustration by Patrick Lane, ScEYEnce Studios.
Expansion of hematopoietic stem and progenitor cells (HSPCs) has been used to increase the low numbers of HSPCs in banked umbilical cord blood (UCB) units2 and for ex vivo genetic engineering of HSPCs for gene therapy.3 A wide range of approaches has been used for the ex vivo expansion of HSPCs, including cytokine cocktails (sometimes in combination with additional small molecules), ectopic expression of specific genes, and coculture with stromal cells.4 One of the recurrent problems has been the progressive loss of stemness upon expansion. For example, the cocktail of stem cell factor (SCF), thrombopoietin (TPO), and Flt3L, often used in ex vivo gene-therapy protocols, was chosen because it minimizes the loss of stem cells. During the last few years, several small molecules have shown promising results in combination with cytokines in promoting HSPC expansion.4
Lysine acetylation is a fundamental posttranslational modification that plays an important role in the control of gene transcription in chromatin.5 BET proteins (BRD2, BRD3, BRD4) are ubiquitously expressed proteins containing an N-terminal bromodomain (BRD) that binds to acetylated lysine residues in histones.5 They can be regarded as epigenetic readers and transcriptional cofactors and are often found in superenhancers that direct cell-lineage fate decisions. CPI203 is a small molecule inhibitor that specifically targets BRD4 by competitive binding to the acetyl-lysine recognition pocket, thereby displacing BRD4 from chromatin. BRD4 marks the transcription start sites of cell-cycle genes. It also promotes progression of the cell cycle from G1 to S and G2 to M phase and has a critical role in hematopoiesis.6
BET proteins play an important role in regulating HSC stemness in the mouse. For instance, a BET inhibitor termed JQ1, which is structurally related to CPI203, increases HSC proliferation and mobilization in mice.7 Based on these results, Hua et al from Oxford’s Institute of Molecular Medicine, investigated 10 known small molecule inhibitors of these proteins, by adding them in increasing concentration to stem cell expansion cultures containing SCF, TPO, and Flt3L, which by itself cannot maintain or expand phenotypically or functionally defined human HSCs. Addition of only 1 of them, CPI203, resulted in significant expansion of human Lin−CD34+CD38−CD45RA−CD90+CD49f+ cells, designated as phenotypical HSCs (pHSCs). Cell viability, a problem that was seen with some other compounds, was not affected.
The investigators showed that although the total number of nucleated cells was lower, the numbers of immature pHSCs were higher and had actually expanded in the CPI203-supplemented cultures (see figure). In addition, they calculated that the total numbers of long-term culture-initiating cells in the pHSC subset was increased fivefold to 10-fold. Additional limiting dilution transplantation experiments in nonirradiated immune-deficient NSG and NSG-W mice indicated multilineage reconstitution and bone marrow engraftment levels up to 23% compared with <1% for control mice transplanted with cells expanded with cytokines only. The preservation of HSC function and self-renewal was further confirmed in secondary transplantation experiments.
Another remarkable observation was the strong increase in CD41+CD42+ MKs in their cultures (see figure). This occurred due to increased differentiation from committed progenitors and, perhaps also, by altering the lineage potential of HSCs. The potential alteration of stem cell lineage fate is an important and as yet unexplored issue of the current study. In addition, the number of developing T lymphocytes is low under the conditions tested and other reports have shown that T-cell function can be inhibited by BET inhibitors.8 Therefore, the current promising data raise a number of critical questions. What are the molecular mechanisms underlying expansion of LT-HSCs and how is this linked to the selective promotion of MK differentiation? Some studies have indicated that BET inhibition increases β-catenin/Wnt signaling,9 which would not only explain the HSC effects but also the reported effects on MKs.10
Although mechanistic insight is still limited, the authors have made the important observation of expansion of long-term repopulating stem cells and promotion of MK development. The clinical translation of these observations, both with respect to their effects on HSCs and MK development will require a more complete analysis of the hematopoietic effects of CPI203. Their effects on B-cell and T-cell development should be explored in more detail. Is CPI203 involved in stem cell mobilization, as suggested for other BET inhibitors used in mice? Altogether, the study holds potential promise for the application of CPI203 in protocols to increase limited numbers of stem cells, for instance in UCB transplantation, in which HSC numbers are limited, and also in gene therapy and gene-editing protocols that rely on in vitro genetic modification.
Conflict-of-interest disclosure: The authors declare no competing financial interests.