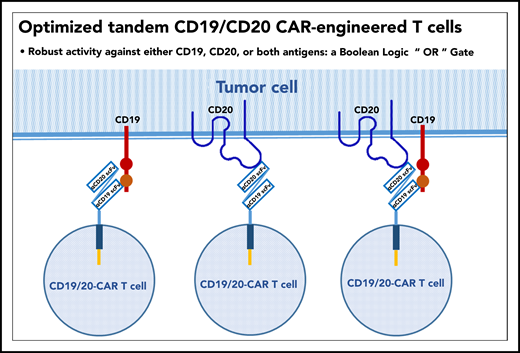
In this issue of Blood, Tong et al present the preclinical and clinical development of an optimized, bivalent tandem CD20/CD19 chimeric antigen receptor (CAR) construct (see figure). Preclinically, they demonstrated dual-antigen specificity, enhanced immune synapse formation, and superior antitumor activity in vitro and in vivo in a murine xenograft model compared with alternative constructs generated from the same 2 single-chain variable fragment (scFv) regions derived from Leu-16 (anti-CD20) and FMC63 (anti-CD19) murine monoclonal antibodies. Next, they performed a phase 1/2a clinical trial of this construct in patients with relapsed or refractory mature B-cell lymphomas or chronic lymphocytic leukemia.1 Their observations suggest that a single chimeric receptor targeting 2 separate tumor antigens is a safe and potentially effective CAR T-cell approach with potential to prevent therapeutic failure due to single antigen loss by tumor cells after CD19-directed therapies as well as to enhance the functional activity of the CAR in T cells.
Single chimeric antigen receptor with tandem CD19 and CD20 specificities.
Single chimeric antigen receptor with tandem CD19 and CD20 specificities.
CAR T-cell therapy directed against CD19 has changed the prognosis for relapsed/refractory aggressive large B-cell lymphomas. There are now 2 commercially available products for this indication: axicabtagene ciloleucel, approved in the United States in late 2017 based on the ZUMA-1 trial,2 and tisagenlecleucel, previously approved in the United States in 2017 for relapsed/refractory pediatric/young-adult B-cell acute lymphoblastic leukemia (B-ALL) and later approved in the United States in early 2018 for the relapsed/refractory diffuse large B-cell lymphoma indication based on the JULIET trial.3 In these pivotal trials, long-term disease-free survival of treated patients was between 30% and 40%, a significant improvement over historical outcomes but clearly with room for improvement. In addition, a plateau in survival curves emerged beyond 6 months; most therapeutic failures occurred within the first 3 months of therapy despite evidence of in vivo expansion of CAR T cells. With this new therapeutic approach established, the race began to understand the mechanisms of resistance to CAR T cells and to reengineer CARs based on the identified mechanisms of resistance. Currently, the development of next-generation CARs spans the discovery and preclinical and clinical phases of development. In addition, alternative strategies that combine immunomodulatory small molecules and monoclonal antibodies with CAR T cells to overcome resistance mechanisms and enhance effectiveness are in development.4
Among the first observations to suggest a mechanism of resistance to CD19-directed CAR T cells in B-cell lymphoma patients was loss of the CD19 antigen by tumor cells in biopsies obtained after treatment, a phenomenon occurring in ∼20% to 27% of treatment failures and previously observed in cases of relapsed B-ALL.2,5,6 To address the issue of single-target antigen escape, a number of approaches aimed at targeting multiple antigens have been pursued in laboratory studies and in clinical trials, including the following:
Combining or sequentially administering 2 CAR T-cell products that each express scFvs with different antigenic specificities;
Simultaneously transducing T cells with 2 CAR vectors that each contain an scFv with different antigenic specificities;
Transducing T cells with a single CAR vector encoding 2 unique CARs separated by a ribosomal skip sequence to generate so-called bicistronic CAR T cells, which express separate chimeric receptors with different antigen-specificities; and
Transducing T cells with a single CAR vector that expresses 2 scFvs with different antigen specificities on a single receptor to generate so-called tandem CAR T cells.7
Recently, 2 large clinical trials of the first approach, using sequential administration of CD19 and CD22 CAR T cells, in patients with refractory/relapsed B-cell malignancies and pediatric refractory/relapsed B-ALL were reported.8,9 Both trials showed that this approach is safe and efficacious with apparently durable responses that may reflect reduction of antigen escape. Cumulatively, only 4 patients with B-ALL had loss or downregulation of CD19 and/or CD22 at the time of relapse after infusion. Loss of CD19 or CD22 was not observed in any patient with B-cell lymphoma at relapse.
Using the tandem CAR approach, Tong and colleagues developed a single CAR construct that coexpressed receptors for both CD19 and CD20. This construct also conferred enhanced immune synapse formation, a favorable cytokine profile, and additive antitumor activity compared with monovalent anti-CD19 and anti-CD20 CAR constructs in preclinical studies. This preclinical work was tested in a phase 1/2a clinical trial in 28 patients with a variety of relapsed/refractory mature B-cell neoplasms. The overall response rate was 79%, complete response rate was 71%, and progression-free survival at 12 months was 64%. Cytokine release syndrome was similar to other 4-1BB CARs with 14% grade 3 events; no grade 3 or higher neurotoxicity was observed.
Although these early results are certainly promising and may reflect reduction in therapeutic failures related to antigen escape, additional studies with larger disease-specific cohorts are needed to compare outcomes of tandem bispecific CAR T cells with currently available CAR T-cell therapies. However, if we assume that “Two heads are better than one,” the road forward for NextGen CARs seems clear.10
Conflict-of-interest disclosure: S.J.S. reports personal fees related to advisory boards and/or consulting from Acerta, AstraZeneca, Celgene, BeiGene, Loxo Oncology, AlloGene, Novartis, Genentech/Hoffman-La Roche, and Tessa Therapeutics; he also reports the following patent “COMBINATION THERAPIES OF CHIMERIC ANTIGEN RECEPTORS AND PD-1 INHIBITORS” with royalties paid to Novartis.