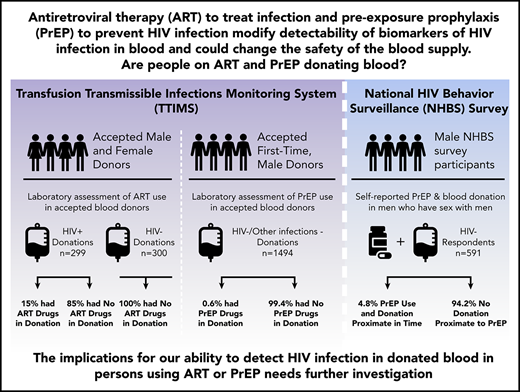
Key Points
Fifteen percent of HIV-positive donors in the United States took ART within a few days before donating.
PrEP use proximate to donation was found in 0.6% of first-time male blood donors and by survey in 5% of MSM.
Abstract
Antiretroviral therapy (ART) to treat and pre-exposure prophylaxis (PrEP) to prevent HIV infection are effective tools to help end the HIV epidemic. However, their use could affect HIV transfusion-transmission risk. Three different ART/PrEP prevalence analyses in blood donors were conducted. First, blood samples from HIV-positive and a comparison group of infection-nonreactive donors were tested under blind using liquid chromatography-tandem mass spectrometry for ART. Second, blood donor samples from infection-nonreactive, 18- to 45-year-old, male, first-time blood donors in 6 US locations were tested for emtricitabine and tenofovir. Third, in men who have sex with men (MSM) participating in the 2017 Centers for Disease Control and Prevention National HIV Behavioral Surveillance (NHBS) from 5 US cities, self-reported PrEP use proximate to donation was assessed. In blind testing, no ART was detected in 300 infection-nonreactive donor samples, but in 299 HIV confirmed-infected donor samples, 46 (15.4%; 95% confidence interval [CI], 11.5% to 20.0%) had evidence of ART. Of the 1494 samples tested from first-time male donors, 9 (0.6%; 95% CI, 0.03% to 1.1%) had tenofovir and emtricitabine. In the NHBS MSM survey, 27 of 591 respondents (4.8%; 95% CI, 3.2% to 6.9%) reported donating blood in 2016 or 2017 and PrEP use within the same time frame as blood donation. Persons who are HIV positive and taking ART and persons taking PrEP to prevent HIV infection are donating blood. Both situations could lead to increased risk of HIV transfusion transmission if blood screening assays are unable to detect HIV in donations from infected donors.
Introduction
There has been enormous progress in reducing the risk of transfusion-transmitted (TT) HIV through donor deferral policies and advances in serological and molecular (nucleic acid testing [NAT]) assays.1-3 The risk of TT-HIV is now <1 in 1 million transfusions in the United States and other developed countries.4 Globally, the small number of documented NAT breakthrough TT cases (∼25 cases reported since implementation of NAT beginning in the late 1990s) have been attributed to early problems with commercial blood screening assay design, defects during test kit manufacture, or very low–viral load (VL) infectious window–phase donations.4 The great majority of TT-HIV cases have been from low-VL infectious window–phase donations.5-7 Even so, documented TT-HIV probably underrepresents actual transmission due to failure to diagnose or report infections, and reported TT-HIV is an order of magnitude lower than that predicted by current risk estimates.
The current high confidence in the safety of the blood supply for HIV achieved through screening is now being revisited.8,9 The cause for concern is the potential impact of interventions to reduce HIV through antiretroviral (ARV) therapies (ARTs) following HIV diagnoses and widespread availability of postexposure prophylaxis and pre-exposure prophylaxis (PrEP), which are highly effective when taken as prescribed.10-14 HIV PrEP awareness and use in at-risk populations has steadily increased since 2013.15 With the recent approval of a second drug combination for PrEP and the anticipated generic availability of the first therapy, use is likely to continue to increase. These initiatives have high-level scientific and political endorsement coupled with media promotion of the concept that early and sustained therapeutic ART and PrEP effectively prevent sexual transmission of HIV; eg, the “Undetectable Equals Untransmittable” campaign.16 Recent years have seen expansion of domestic and international programs most recently promulgated as the US government’s “End the HIV Epidemic” initiative (https://www.hiv.gov/federal-response/ending-the-hiv-epidemic/overview).
Use of ART causes HIV-infected persons to exhibit modified biomarkers of infection, such as undetectable RNA by NAT/VL assays, and may result in undetectable antibodies by third- and fourth-generation serological screening assays.9,17 In addition to suppression of viremia, ART is known to alter biomarkers of HIV infection progression and may result in antibody “seroreversion”; the latter may impact the ability to detect HIV infection through current blood donation screening. Studies of persons treated early show that they remain infected based on findings from analytical treatment interruption studies, with all early-treated individuals demonstrating rebounding viremia weeks to months following analytical treatment interruption.18,19 Blood donated by early-treated individuals could test negative for HIV RNA and antibodies (and even ARVs if they had interrupted ART use) and potentially transmit HIV infection to transfusion recipients given the large volume infused during transfusion. Furthermore, studies of persons on PrEP have documented suppressed VLs and delayed seroconversion in confirmed breakthrough infections,20 presenting challenges to detecting and confirming PrEP breakthrough HIV infection and evaluating PrEP efficacy in clinical trials and following implementation.11,21 In addition, reports documenting HIV-specific cellular immune responses suggest that abortive or latent infections may occur while on PrEP.22,23
Prospective blood donors undergo an eligibility assessment when they present to give blood. This assessment includes a donor history questionnaire (DHQ). Along with a brief physical examination for overt medical contraindications for donation, DHQ responses are reviewed with a health professional to assess if risks are present that justify excluding the donor from giving blood. The DHQ used in each country is informed by the country’s regulatory requirements and risk tolerance and is tailored to the perceived or known risk behaviors associated with transfusion-transmissible infections or other risks prevalent in that setting.24 AABB (formerly the American Association of Blood Banks) develops the DHQ used in the United States, and the version in use at the time of this study was released in February 2016. The questions on the US DHQ inquire about sexual behavior risk, specific disease risk, other medical history relevant to blood transfusion risk, and travel to areas with risk for some transfusion-transmissible infections (eg, malaria and babesia). At the time of this study, the DHQ included direct queries about male-male sex within the past 12 months, having ever tested positive for the HIV/AIDS virus, and if a person was taking any medication to treat an infection or any medication on the medication deferral list. This list changes as new information emerges. Also at the time of this study, the US DHQ did not ask whether a person was taking medication to prevent an HIV infection (ie, PrEP) or list PrEP trade names on the medication deferral list. The prevalence of persons taking ARV drugs is expected to increase considering treatment guidelines for HIV that recommend starting ARVs immediately upon confirmed diagnosis and expanded prevention campaigns to increase availability of postexposure prophylaxis and PrEP. The objective of the present set of studies was to assess if there is evidence of ARV and PrEP use in blood donors in the United States.
Methods
Study design
In 2015, the US Food and Drug Administration (FDA); the National Institutes of Health/National Heart, Lung, and Blood Institute (NHLBI); and the Office of the Assistant Secretary of Health, Department of Health and Human Services funded the Transfusion-Transmissible Infections Monitoring System (TTIMS).25 The program was implemented to monitor known and emerging blood safety topics, such as the change from an indefinite deferral from blood donation by men who have sex with men (MSM) to a 12-month deferral since last sex,26 which has now been reduced to a 3-month deferral since last sex.27 TTIMS uses standardized procedures for collecting demographic and test result data for ∼60% (almost 7 million per year) of voluntary blood donations collected in all or part of the contiguous 48 states and District of Columbia. TTIMS also includes a biospecimen repository of plasma aliquots from donors with HIV, hepatitis C virus, or hepatitis B virus infections, as identified through blood donation testing (these donations are discarded [ie, not transfused] and the donors deferred from future donations). The participating blood centers are the American Red Cross, Vitalant, OneBlood, and the New York Blood Center. These blood collection organizations implemented the 12-month deferral policy for MSM in the period from 8 August to 12 December 2016.
ART use in donors
The 4 blood collection organizations provided individual donor plasma samples from HIV confirmed-positive whole-blood donations identified through minipool NAT (16 samples per minipool) and serology testing from September 2015 through December 2017. The HIV-positive samples were tested using the Hologic HIV-1 RNA Aptima VL assay, which has a 95% limit of detection of 12 copies/mL and lower limit of quantitation (LLOQ) of 30 copies/mL (Hologic, San Diego, CA). Donation samples from HIV confirmed-positive donors were tested. To assess the performance of the assay a randomly selected sample from donors who screened infection-nonreactive for all markers of infection (HIV-1/2, hepatitis B virus, hepatitis C virus, human T-lymphotropic virus type I/II, West Nile virus, Zika virus, Treponema pallidum, and Trypanosoma cruzi) and who would not be expected to be reactive for ARVs were also tested. Samples were held at −80°C until tested. The testing laboratory at the CDC Division of HIV/AIDS Prevention that performed ARV testing was blinded to the infection status of each specimen.
Thirteen ARV drug analytes (raltegravir, tenofovir [TFV], abacavir, ritonavir, lamivudine, efavirenz, emtricitabine [FTC], elvitegravir, dolutegravir, cobicistat, etravirine, darunavir, and rilpivirine) were simultaneously measured by high-performance liquid chromatography-tandem mass spectrometry (Sciex, Foster City, CA; Shimadzu Scientific, Columbia, MD). Details of the analytical procedures and estimated plasma half-lives in hours for each ARV are provided in supplemental Table 1 (available on the Blood Web site). Estimates of the day of last ART drug ingestion were determined by constructing approximate pharmacokinetic curves based on published values of maximum serum concentration and half-lives for each analyte in plasma (fact sheets found at http://hiv-druginteractions.org). Data for TFV are for tenofovir disoproxil fumarate–derived TFV, not tenofovir alafenamide–derived TFV. Experimental values were then compared with appropriate curves and day of last dose estimated.
PrEP use in donors
Deidentified samples were collected from 1 September 2018 to 31 May 2019. To increase the likelihood of testing samples from donors taking PrEP, we focused on donations from 6 metropolitan centers with above-average access to PrEP: Boston, Los Angeles, Miami, New York City, San Francisco, and Washington, DC.28 Donations from zip codes of residence in the areas of each city where the prevalence of diagnosed HIV is >1000 per 100 000 male residents29 were selected. Consecutive specimens from male, first-time donors 18 to 45 years of age were obtained after testing nonreactive for all routinely screened infections. The same CDC laboratory testing methods were used to assess presence and days since use of TFV and FTC, the 2 drugs in Truvada.
NHBS survey to assess PrEP use and blood donation
The National HIV Behavioral Surveillance (NHBS) survey is conducted by the US CDC Division of HIV/AIDS Prevention in collaboration with state and city departments of health in >20 cities in the United States. Surveys are repeated in cycles every 3 years in higher risk groups. The sampling methods for MSM in NHBS have been described previously.30-32 Briefly, time-location sampling is used to randomly select venues frequented by MSM and recruit participants consecutively. Venues include bars, dance clubs, parks, cafes, street locations, and social organizations (ie, gay softball leagues or community groups). Men are eligible if ≥18 years, attending a venue that was randomly selected, and reported ever having anal or oral sex with a man. Verbal consent is obtained, an interviewer-administered survey is conducted, and a blood sample provided for HIV antibody testing. A short set of questions related to blood donation was added to the 2017 MSM cycle 5 (MSM5) in 5 sites: Atlanta, Los Angeles, New Orleans, Seattle, and San Francisco. Field interviews for these sites were conducted during the period of June to December 2017. Data analyses were conducted in SAS v 9.4 (Cary, NC) locally at each participating NHBS site using a common data analysis program code provided to all. An unweighted overall proportion of MSM taking PrEP and reporting blood donation in 2016 or 2017 was estimated.
Ethics approval and informed consent
The ART and PrEP study procedures were approved by institutional review boards at the University of California, San Francisco; the American Red Cross; the New York Blood Center; and the FDA. Blood donors provided consent for the use of donation data and biospecimens in blood safety research at the time of donation. The NHBS survey procedures were approved by institutional review boards at each of the participating NHBS locations.
Results
ART use in donors
We assessed ARV drugs in samples from 299 donations from HIV confirmed-positive donors from the period of 1 September 2015 to 31 December 2017 and from 300 infection-nonreactive donors from 12 September 2018 to 11 November 2018 (Table 1). All infection-nonreactive donor specimens were below the LLOQ for each of the 13 ARV drugs analyzed. Of the samples from HIV confirmed-positive donors, 46 (15.4%; 95% confidence interval [CI], 11.5% to 20.0%) had evidence of ARVs, with 45 having >1 drug identified. Detected ARVs were consistent with several commonly prescribed therapies (supplemental Table 2).
ART detection in 299 HIV-positive voluntary blood donations collected in the United States from September 2015 through December 2017 and 300 infection-nonreactive donations collected from September through November 2018
| HIV blood screening results . | HIV-positive donors at TTIMS blood centers during period* . | Samples tested for ARVs . | ARVs detected n (%) . | Estimated days since last ARV dose, n (row %) . | ||
|---|---|---|---|---|---|---|
| 1 d ago . | 2 d ago . | 3 d ago . | ||||
| HIV negative | — | 300 | 0 | |||
| HIV positive | 463 | 299 | 46 (15.4)† | 31 (67.4) | 12 (26.1) | 3 (6.5) |
| NAT yield (NAT reactive, serology nonreactive | 11 | 0 | — | — | — | — |
| NAT and serology reactive | 398 | 252 | 5 (2.0) | 4 (80.0) | 1 (20.0) | 0 |
| Serology reactive | 54 | 47 | 41 (87.3) | 27 (65.9) | 11 (26.8) | 3 (7.3) |
| HIV blood screening results . | HIV-positive donors at TTIMS blood centers during period* . | Samples tested for ARVs . | ARVs detected n (%) . | Estimated days since last ARV dose, n (row %) . | ||
|---|---|---|---|---|---|---|
| 1 d ago . | 2 d ago . | 3 d ago . | ||||
| HIV negative | — | 300 | 0 | |||
| HIV positive | 463 | 299 | 46 (15.4)† | 31 (67.4) | 12 (26.1) | 3 (6.5) |
| NAT yield (NAT reactive, serology nonreactive | 11 | 0 | — | — | — | — |
| NAT and serology reactive | 398 | 252 | 5 (2.0) | 4 (80.0) | 1 (20.0) | 0 |
| Serology reactive | 54 | 47 | 41 (87.3) | 27 (65.9) | 11 (26.8) | 3 (7.3) |
September 2015 through December 2017.
95% CI, 11.5% to 20.0%.
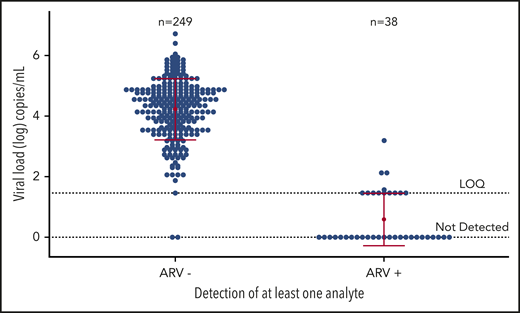
Of the 46 ARV-positive samples, 43 (93.5%) were from first-time donors and 34 (74%) were from males (Table 2). First-time and 45- to 54-year-old HIV-positive donors were significantly more likely to have evidence of ARV use than repeat or younger donors (each P < .001). When we stratified the analysis, the proportion of HIV-positive donors who donated while taking ARVs was not different by sex or race/ethnicity or donation location. HIV VL values in plasma from donors taking ART were significantly lower than for donors not taking ART, demonstrating overall good virologic control (Figure 1).
Demographic characteristics of HIV-positive persons with and without evidence of ARV use at the time of blood donation from 299 HIV-positive voluntary blood donations collected in the United States from September 2015 through December 2017 and 300 infection-nonreactive donations collected from September through November 2018
| Characteristic . | Total . | ARV−, n (%) . | ARV+, n (%) . | χ2 P value . |
|---|---|---|---|---|
| Sex | .56 | |||
| Female | 68 | 56 (82.4) | 12 (17.6) | |
| Male | 231 | 197 (85.3) | 34 (14.7) | |
| Age (y) | <.001 | |||
| 16-17 | 15 | 14 (93.3) | 1 (6.7) | |
| 18-24 | 88 | 83 (94.3) | 5 (5.7) | |
| 25-34 | 80 | 69 (86.3) | 11 (13.8) | |
| 35-44 | 47 | 37 (78.7) | 10 (21.2) | |
| 45-54 | 47 | 31 (66.0) | 16 (33.0) | |
| ≥55 | 22 | 19 (86.4) | 3 (13.6) | |
| Race/ethnicity | .19 | |||
| American Indian | 1 | 1 (100) | 0 | |
| Asian | 6 | 6 (100) | 0 | |
| Black | 119 | 95 (79.8) | 24 (20.2) | |
| Hispanic | 59 | 52 (88.1) | 7 (11.9) | |
| White | 96 | 86 (89.6) | 10 (10.4) | |
| >1 race | 5 | 3 (0.6) | 2 (0.4) | |
| Other/unknown | 13 | 10 (76.9) | 3 (23.1) | |
| Donation history | <.001 | |||
| First time | 203 | 160 (78.9) | 43 (21.1) | |
| Repeat | 96 | 93 (96.9) | 3 (3.1) | |
| Donation location | .81 | |||
| Fixed clinic | 29 | 25 (86.2) | 4 (13.8) | |
| Mobile site | 270 | 228 (84.4) | 42 (15.6) |
| Characteristic . | Total . | ARV−, n (%) . | ARV+, n (%) . | χ2 P value . |
|---|---|---|---|---|
| Sex | .56 | |||
| Female | 68 | 56 (82.4) | 12 (17.6) | |
| Male | 231 | 197 (85.3) | 34 (14.7) | |
| Age (y) | <.001 | |||
| 16-17 | 15 | 14 (93.3) | 1 (6.7) | |
| 18-24 | 88 | 83 (94.3) | 5 (5.7) | |
| 25-34 | 80 | 69 (86.3) | 11 (13.8) | |
| 35-44 | 47 | 37 (78.7) | 10 (21.2) | |
| 45-54 | 47 | 31 (66.0) | 16 (33.0) | |
| ≥55 | 22 | 19 (86.4) | 3 (13.6) | |
| Race/ethnicity | .19 | |||
| American Indian | 1 | 1 (100) | 0 | |
| Asian | 6 | 6 (100) | 0 | |
| Black | 119 | 95 (79.8) | 24 (20.2) | |
| Hispanic | 59 | 52 (88.1) | 7 (11.9) | |
| White | 96 | 86 (89.6) | 10 (10.4) | |
| >1 race | 5 | 3 (0.6) | 2 (0.4) | |
| Other/unknown | 13 | 10 (76.9) | 3 (23.1) | |
| Donation history | <.001 | |||
| First time | 203 | 160 (78.9) | 43 (21.1) | |
| Repeat | 96 | 93 (96.9) | 3 (3.1) | |
| Donation location | .81 | |||
| Fixed clinic | 29 | 25 (86.2) | 4 (13.8) | |
| Mobile site | 270 | 228 (84.4) | 42 (15.6) |
HIV-1 RNA concentrations in ARV-negative and ARV-positive blood donors. A comparison of HIV RNA concentrations in ARV-negative and ARV-positive donations from HIV-positive persons with and without evidence of ART use at the time of blood donation from 299 HIV-positive voluntary blood donations collected in the US from September 2015 through December 2017. Of the 299, 287 samples had sufficient volume for viral load testing. Each mean is represented by a red dot and 1 standard deviation by the horizontal red lines. LOQ, limit of quantitation.
HIV-1 RNA concentrations in ARV-negative and ARV-positive blood donors. A comparison of HIV RNA concentrations in ARV-negative and ARV-positive donations from HIV-positive persons with and without evidence of ART use at the time of blood donation from 299 HIV-positive voluntary blood donations collected in the US from September 2015 through December 2017. Of the 299, 287 samples had sufficient volume for viral load testing. Each mean is represented by a red dot and 1 standard deviation by the horizontal red lines. LOQ, limit of quantitation.
PrEP use in donors
We found evidence of PrEP use in donation samples from donors who were infection-nonreactive, including for HIV, from the targeted population of male, first-time donors. Of 1494 samples tested, 9 (0.6%; 95% CI, 0.03% to 1.1%) had detectable levels of both TFV and FTC (Table 3). Specimens from 5 of the 6 metropolitan areas had evidence of PrEP, and 5 of the 9 donors were estimated to have last taken PrEP medication within 2 days of donating.
Evidence of PrEP use in first-time male voluntary blood donors from deidentified samples collected between 1 September 2018 and 31 May 2019 from 6 metropolitan locations
| Geographic location . | Samples . | PrEP drugs detected, n (%) . | Estimated days since last ARV dose, n (%) . | ||
|---|---|---|---|---|---|
| 1 d ago . | 2 d ago . | 3 d ago . | |||
| Boston, MA | 176 | 0 (0) | |||
| Los Angeles, CA | 344 | 2 (0.6) | 0 | 0 | 2 (100) |
| Miami, FL | 243 | 1 (0.4) | 0 | 1 (100) | 0 |
| New York, NY | 350 | 2 (0.6) | 0 | 0 | 2 (100) |
| San Francisco, CA | 211 | 2 (1.0) | 1 (50.0) | 1 (50.0) | 0 |
| Washington, DC | 170 | 2 (1.2) | 2 (100) | 0 | 0 |
| Total | 1494 | 9 (0.6)* | 3 (33.3) | 2 (22.2) | 4 (44.4) |
| Geographic location . | Samples . | PrEP drugs detected, n (%) . | Estimated days since last ARV dose, n (%) . | ||
|---|---|---|---|---|---|
| 1 d ago . | 2 d ago . | 3 d ago . | |||
| Boston, MA | 176 | 0 (0) | |||
| Los Angeles, CA | 344 | 2 (0.6) | 0 | 0 | 2 (100) |
| Miami, FL | 243 | 1 (0.4) | 0 | 1 (100) | 0 |
| New York, NY | 350 | 2 (0.6) | 0 | 0 | 2 (100) |
| San Francisco, CA | 211 | 2 (1.0) | 1 (50.0) | 1 (50.0) | 0 |
| Washington, DC | 170 | 2 (1.2) | 2 (100) | 0 | 0 |
| Total | 1494 | 9 (0.6)* | 3 (33.3) | 2 (22.2) | 4 (44.4) |
All samples with PrEP detected had with measurable levels of both TFV and FTC.
95% CI, 0.03% to 1.1%.
NHBS survey to assess PrEP use and blood donation
The analysis of the 2017 NHBS MSM survey showed that 27 out of 565 HIV-negative respondents (4.8%; 95% CI, 3.2% to 6.9%) reported donating blood in 2016 or 2017 with PrEP use proximate to the time of donation (Table 4). Respondents with history of PrEP use and blood donation were seen in all 5 NHBS sites in this analysis. Demographics of the subset of MSM who reported blood donation in 2016 or 2017 after PrEP use are reported in supplemental Table 3. Similar to the assessment of PrEP use in known blood donors, >90% of NHBS respondents reporting PrEP use and history of blood donation were between the ages of 20 and 39 years.
PrEP and blood donation during 2016 and 2017 based on responses by HIV-negative respondents to the NHBS among MSM in Atlanta, Los Angeles, New Orleans, Seattle, and San Francisco during field interviews conducted between June 2017 and December 2017
| Topic . | Atlanta (n = 399) . | Los Angeles (n = 442) . | New Orleans (n = 307) . | Seattle (n = 447) . | San Francisco (n = 399) . | Total (N = 1994) . |
|---|---|---|---|---|---|---|
| Tried to donate blood since first having had oral or anal sex with a man | ||||||
| No | 270 (67.7) | 293 (66.3) | 156 (50.8) | 283 (63.3) | 282 (70.7) | 1282 (64.3) |
| Yes | 86 (21.6) | 127 (28.7) | 120 (39.1) | 122 (27.3) | 110 (27.6) | 565 (28.3) |
| Missing | 43 (10.8) | 22 (5.0) | 31 (8.4) | 42 (9.4) | 7 (1.8) | 145 (7.3) |
| In what year did you last donate blood?* | ||||||
| 2015 or before | 51 (59.3) | 94 (74.0) | 100 (83.3) | 78 (63.9) | 94 (85.5) | 417 (73.4) |
| 2016 or 2017 | 18 (20.9) | 21 (16.5) | 20 (16.7) | 14 (11.5) | 8 (7.3) | 81 (14.3) |
| Missing | 17 (19.8) | 12 (9.4) | — | 30 (24.6) | 8 (7.3) | 67 (11.8) |
| PrEP use in the 12 mo preceding participation in NHBS MSM in 2017† | ||||||
| Yes | 4 (22.2) | 6 (28.6) | 7 (35.0) | 5 (35.7) | 5 (62.5) | 27 (33.3) |
| No | 12 (60.0) | 12 (57.1) | 12 (60.0) | 8 (57.1) | 2 (25.0) | 43 (53.0) |
| Don’t know/missing | 1 (5.0) | 3 (14.2) | 1 (5.0) | 1 (7.1) | 1 (12.5) | 11 (13.4) |
| Topic . | Atlanta (n = 399) . | Los Angeles (n = 442) . | New Orleans (n = 307) . | Seattle (n = 447) . | San Francisco (n = 399) . | Total (N = 1994) . |
|---|---|---|---|---|---|---|
| Tried to donate blood since first having had oral or anal sex with a man | ||||||
| No | 270 (67.7) | 293 (66.3) | 156 (50.8) | 283 (63.3) | 282 (70.7) | 1282 (64.3) |
| Yes | 86 (21.6) | 127 (28.7) | 120 (39.1) | 122 (27.3) | 110 (27.6) | 565 (28.3) |
| Missing | 43 (10.8) | 22 (5.0) | 31 (8.4) | 42 (9.4) | 7 (1.8) | 145 (7.3) |
| In what year did you last donate blood?* | ||||||
| 2015 or before | 51 (59.3) | 94 (74.0) | 100 (83.3) | 78 (63.9) | 94 (85.5) | 417 (73.4) |
| 2016 or 2017 | 18 (20.9) | 21 (16.5) | 20 (16.7) | 14 (11.5) | 8 (7.3) | 81 (14.3) |
| Missing | 17 (19.8) | 12 (9.4) | — | 30 (24.6) | 8 (7.3) | 67 (11.8) |
| PrEP use in the 12 mo preceding participation in NHBS MSM in 2017† | ||||||
| Yes | 4 (22.2) | 6 (28.6) | 7 (35.0) | 5 (35.7) | 5 (62.5) | 27 (33.3) |
| No | 12 (60.0) | 12 (57.1) | 12 (60.0) | 8 (57.1) | 2 (25.0) | 43 (53.0) |
| Don’t know/missing | 1 (5.0) | 3 (14.2) | 1 (5.0) | 1 (7.1) | 1 (12.5) | 11 (13.4) |
Values are presented as n (%) of respondents.
Among those who tried to donate blood (n = 565).
Among those who donated in 2016 and 2017 (n = 81).
Discussion
We document compelling evidence of ART and PrEP use in blood donors in the United States. First, we found HIV-positive donors donating with ARVs in their donations. Second, 2 different approaches found evidence of PrEP use in HIV-negative male blood donors: direct testing for PrEP in donation samples and survey participants reporting PrEP use proximate to donation. The implications for blood safety are currently unknown. These donations have the potential to lead to TT-HIV if viremia and antibody levels are sufficiently low to be undetectable by the assays used to screen blood. Identification of ARVs and PrEP in blood donations prompt the need for further investigation to assess the extent of risk to blood recipients.
Outside of the blood donation context, in recognized HIV risk groups, several cases of NAT-yield infections with very low VLs that contain PrEP drugs have been identified; on longitudinal follow-up, these persons did not develop high-level viremia or HIV antibodies until they discontinued PrEP.33-35 Adherence to daily dosing of PrEP is challenging and directly related to prevention efficacy.36,37 Moreover, as approaches to PrEP use evolve, including use of “on-demand” PrEP when sexual exposure is expected, concern about imperfect adherence leading to breakthrough infection in transfusion may increase.
Based on testing NAT-nonreactive and antibody-confirmed positive during routine donation screening, nearly 90% of the ARV-positive donors would be considered potential HIV elite controllers (defined as people living with HIV without taking ART who have a VL near or below the limit of detection for long periods) if the ARV testing results were not available.38 The serological assay used to screen donations during this study period (third-generation HIV-1/HIV-2 chemiluminescence immunoassay, PRISM; Abbott Laboratories, Abbott Park, IL) was able to detect the serology-only HIV infections, with the majority of these samples demonstrating high-level antibody reactivity (supplemental Figure 1). These findings suggest that these donors had long-standing infections and had relatively recently initiated ART, since their antibody levels had not yet started to wane as has been documented to occur following initiating ART soon after seroconversion or following prolonged viral suppression.39,40 However, ARVs were detected in 5 donors who tested NAT and serology reactive. These donation samples, all from first-time donors, had quantifiable, albeit relatively low VLs, suggesting recent initiation of therapy, suboptimal therapy, or poor adherence to ART.
Analyses of samples from HIV-positive donors in South Africa document that some persons with HIV infection are on ART and donating blood despite deferral questions that should have precluded selection for donation.41 Similarly, for US donors who know they are HIV positive and taking ARVs, our results raise questions about donor knowledge, effectiveness of donor screening criteria and the donation process itself. The detected ARV concentrations in plasma samples suggest ART use occurred within days before donation. The DHQ in use at the time of these donations had 2 questions that if responded to in the affirmative would have excluded these individuals: a direct question about being HIV positive and a general question about taking any medication to treat an infection. The circumstances that contribute to these individuals donating blood are unknown. Blood donation messaging and predonation education should include information noting that HIV viral suppression through use of ART confers protection to sexual partners (ie, Undetectable Equals Untransmittable), but not necessarily blood transfusion. Donors may have believed the setting for donation, such as at a mobile site, did not provide enough privacy for disclosure of HIV-related topics. Recognition that HIV-positive donors on ART are donating has prompted the FDA to issue a 2019 Safety and Availability Communication reinforcing that persons who have ever tested HIV confirmed positive should not donate blood regardless of ART drug use.42
The evidence from the targeted assessment we conducted that a nonnegligible proportion of blood donors are taking PrEP presents a different set of concerns. These results suggest that some recipients of donated blood components may be exposed to PrEP drugs during transfusion. At the time of this study, donors may not have disclosed PrEP use on the DHQ because there was no direct question about PrEP. DHQ questions asked if a person was taking any medication for an infection, not to prevent an infection. These donors may be making an overall self-assessment of their own perceived low HIV risk and assumed the same low risk would apply to recipients of their donated blood. If true, this way of viewing the DHQ questions as a general assessment of the risk as opposed to the specific content of the questions that are asked is consistent with previous research. A qualitative interview study of the DHQ conducted when MSM were indefinitely deferred from donation examined respondents’ understanding of the questions and assessed if MSM interpreted the DHQ differently. All respondents reported they understood the DHQ was asking a general question; “Is my blood safe to donate?” and this interpretation applied for both accepted donors and MSM who were not donors.43 The donors from our study who are taking PrEP and donating may be making a similar self-assessment.
This study has limitations. One limitation of this study is the LLOQ or ability of the tests used to detect ARV and PrEP drugs in plasma, which can identify ARV or PrEP use up to 3 days before donation. Other human specimens such as whole blood, dried blood spots, or hair can be used to identify ARV drugs for an extended period of time and have been used to monitor adherence.44,45 Some donors may have used ART or PrEP outside of the 3-day detectable period in this study. Therefore, our estimates of ART and PrEP use are restricted to recent use. This study cannot identify individuals on ART or PrEP with sporadic use or on-demand PrEP use outside of a 3-day period before donation. On-demand or event-drive PrEP use presents additional challenges to blood safety screening.
A second limitation is related to the laboratory assessment of PrEP use in first-time male donors. This pilot assessment was intentionally targeted to 6 cities where availability and public health promotion of PrEP were established during the sample collection period (2018 and 2019). The PrEP testing results are not representative of all first-time male donors throughout the country.
The TTIMS program was developed to monitor the risk of important TT infections. The adoption of the 12-month deferral for blood donation has not increased the incidence of HIV in first-time donors or changed the residual risk of transfusion transmission.46 However, because of ART and PrEP concerns related to blood safety, AABB has updated the DHQ to explicitly ask if medications are being taken to treat or prevent HIV infection, and the brand and generic names of ART and PrEP drugs have been added to the medication deferral list. Donors who disclose PrEP use will be deferred for 3 months if no other risk behaviors are reported; those who disclose ARV use for HIV infection will be indefinitely deferred (as HIV confirmed-positive donors are today).
Overall, these findings of HIV-positive persons donating while taking ART drugs and donors who are using PrEP identify a new area of HIV blood safety research. Specific studies that are planned as part of TTIMS are expanded evaluation of ART in HIV-positive donors over time to assess trends, larger studies of PrEP use in demographically diverse first-time and repeat donors, and qualitative interviews to understand the motivations of persons who are taking ART or PrEP and donating blood. In addition, as part of the NHLBI-funded Recipient Epidemiology and Donor Evaluation Study (REDS-IV-P), protocols to establish the blood donation testing implications are planned. These studies will include (1) evaluation of HIV detection using FDA-licensed and prelicensure versions of HIV donor screening NAT and serological tests using contemporary longitudinal panels from ARV and PrEP treatment cohorts, (2) international assessment of HIV detection in paired whole-blood and plasma samples in high-risk persons on PrEP by NAT/VL assays to understand if whole blood is a better matrix for detecting HIV in the era of widespread ART availability, and (3) direct assessment of multiplexed HIV antigen assays to detect humoral immune responses not detected by commercial assays in persons on ART and PrEP.
Our findings highlight the need to understand reasons for donation, improve systems for reporting PrEP use, and reduce blood donation from persons who are taking ART. These efforts to improve donor disclosure may be insufficient. Strategies to increase the sensitivity of donation testing for HIV, such as individual donation NAT, may be needed. Alternately, pathogen reduction, currently approved for treating platelets and plasma in the United States but not yet available to treat red cells or whole blood, could be relevant to mitigate potential risks associated with ART and PrEP use in donors. To maintain the safety of the blood supply and ensure recipient risk has not increased as anti-HIV interventions become more widely available, additional monitoring is required and, if necessary, modified blood screening interventions could be adopted.
Access to deidentified data may be requested from TTIMS by contacting Brian Custer (bcuster@vitalant.org). CDC NHBS data are not available as public-use data sets.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors gratefully acknowledge those individuals below who contributed directly to TTIMS without whom this program would not be successful: Roberta Bruhn, Eduard Grebe, and Daniel Hindes (Vitalant); Rahima Fayed and Rebecca L. Townsend (ARC); and Valerie Green and Sherri Cyrus (CTS). They would also like to gratefully acknowledge all participants and those individuals who conducted the field work for the NHBS MSM5 at the 5 cities. Without their dedication and effort, the authors would not have been able to include NHBS data in their analysis.
The authors are grateful for funding support (contract HHSF223201510149C) for TTIMS from the FDA Center for Biologics Evaluation and Research, the National Institutes of Health/NHLBI, and the HHS Office of the Assistant Secretary for Health, as well as the study guidance provided by these sponsors.
The content is solely the responsibility of the authors and does not represent the policy of the National Institutes of Health, the FDA, the US Centers for Disease Control and Prevention, or the Department of Health and Human Services.
Authorship
Contribution: B.C. contributed to conception/design of all parts of the study, analysis and interpretation of findings, and writing of the manuscript; C.Q., R.H., and A.M. contributed to conception/design of ART/PrEP testing substudies, analysis and interpretation of findings, and review and approval of the manuscript; M.S. contributed to design of ART/PrEP testing substudies, acquisition of samples, and review and approval of the manuscript; R.R., W.R.S., and D.K. contributed to design of ART/PrEP testing substudies, sample acquisition, interpretation of findings, and review and approval of the manuscript; P.C.W. contributed to sample acquisition, interpretation of findings, and review and approval of the manuscript; S.A.A. and A.E.W. contributed to conception/design of ART/PrEP testing substudies, interpretation of findings, and review of the manuscript; H.F.R. and W.M. contributed to conception/design of the NHBS substudy, analysis and interpretation of findings, and review and approval of the manuscript; W.T.R., S.G., K.S., and C.D.M. contributed to analysis and interpretation of findings of the NHBS substudy and review and approval of the manuscript; and S.A.G., S.L.S., and M.P.B. contributed to conception/design of the ART/PrEP testing substudies, analysis and interpretation of findings, and review and approval of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the steering committee members of the Transfusion-Transmissible Infections Monitoring System appears in “Appendix.”
Correspondence: Brian Custer, Vitalant Research Institute, 270 Masonic Ave, San Francisco, CA 94118; e-mail: bcuster@vitalant.org.
Appendix
The Transfusion-Transmissible Infections Monitoring System is the responsibility of the following individuals: B.C. (Vitalant Research Institute, San Francisco, CA), S.L.S. (American Red Cross, Gaithersburg, MD), D.K. (New York Blood Center, New York, NY), R.R. (OneBlood, St. Petersburg, FL), S.A.A. and A.E.W. (US FDA, Silver Spring, MD), and S.A.G. (NHLBI, Rockville, MD).