In this issue of Blood, articles by Manne et al and Hottz et al highlight platelet hyperactivity in COVID-19–associated pathophysiology.1,2 Although the hallmarks of COVID-19 include a brisk inflammatory response and respiratory symptoms, the hematologic manifestations of this infection have also garnered attention, with thrombotic complications taking center stage.3,4 COVID-19–associated coagulopathy has been characterized by an elevated D-dimer, mild thrombocytopenia, and a prolongation of the activated partial thromboplastin time.5 Alongside these laboratory abnormalities, patients present with increased rates of thrombosis.6 The role of platelets in the thrombotic complications of COVID-19 is explored in these 2 articles, establishing that platelet hyperactivity contributes to the coagulopathy seen in COVID-19.
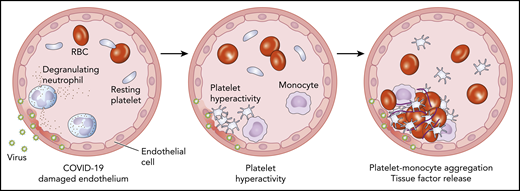
Schematic cross-section of a blood vessel depicting COVID-19–associated endothelial damage (left panel). At the site of endothelial injury, platelets become activated and aggregate (middle panel). These hyperactive platelets activate monocytes, leading to release of tissue factor, the principle regulator of the coagulation cascade (right panel). RBC, red blood cell.
Schematic cross-section of a blood vessel depicting COVID-19–associated endothelial damage (left panel). At the site of endothelial injury, platelets become activated and aggregate (middle panel). These hyperactive platelets activate monocytes, leading to release of tissue factor, the principle regulator of the coagulation cascade (right panel). RBC, red blood cell.
Mounting evidence has demonstrated the far-reaching influence of the platelet beyond mediating hemostasis. Platelets are increasingly recognized as key players in facilitating inflammation. The importance of the platelet in viral infection–mediated thrombosis has been established previously.7 Endothelial damage, a cornerstone of COVID-19 disease, releases key platelet agonists that send platelets into overdrive.8 Inflammation may be exacerbated in patients with hypertension, cardiovascular disease, and obesity, all of which are associated with baseline platelet hyperreactivity.9 Interestingly, studies have shown that patients with these underlying comorbidities suffer more severe COVID-19 complications and have a worse outcome.10 Understanding the role of the platelet in COVID-19 remains elusive. Because the platelet itself does not express the receptor for SARS-CoV-2 binding (ACE2), more remains to be understood about how the virus interacts with platelets and impacts their function.
Propelling the platelet further into the spotlight has been recent evidence demonstrating that megakaryocytes, the precursor cells of the platelet, are present in pulmonary and cardiac tissue from autopsies of patients who have succumbed to COVID-19.11 It remains unclear whether these megakaryocytes in satellite locations contribute to platelet production. Surprisingly, although COVID-19 is associated with severe inflammation, reactive thrombocytosis has not been demonstrated in infected patients. On the contrary, a mild thrombocytopenia is often present, and a decrease in platelet count has been associated with a poor prognosis.12
In the first of 2 linked articles, Manne and colleagues demonstrate changes in platelet gene expression and function in COVID-19 patients.1 Using platelet RNA sequencing, the investigators profile gene expression in the platelets of COVID-19 patients and find altered gene expression profiles in pathways associated with ubiquitination, antigen presentation, and mitochondrial dysfunction. Patients with COVID-19 have higher levels of platelet activation at rest and increased interactions with neutrophils, monocytes, and T cells compared with healthy donors (see figure). Platelet functionality studies demonstrate hyperactivity, as evidenced by increased aggregation, spreading on fibrinogen and collagen through upregulation of the MAPK pathway, and increased thromboxane generation. Therefore, the investigators extrapolate that hyperactivity of platelets plays a role in the immune-mediated thrombotic manifestations seen in patients with COVID-19. What remains to be determined is whether these platelet gene transcript differences and enhanced platelet functionality are uniquely associated with exposure to COVID-19 or whether other similarly inflammatory conditions also reveal comparable changes. Comparisons with other viral illnesses associated with acute respiratory distress syndrome would be helpful in identifying the exclusivity of these platelet manifestations in COVID-19.
In the second article, Hottz and colleagues again demonstrate that COVID-19 is associated with increased platelet activation. They show that the platelets of critically ill COVID-19 intensive care unit (ICU) patients exhibit increased platelet aggregation and platelet-monocyte aggregation compared with patients with mild COVID-19 infection (see figure). These findings are associated with the characteristic coagulation laboratory changes found in patients with COVID-19, including elevated fibrinogen and D-dimer. Further, these changes correlate with a worse outcome, with increased need for invasive mechanical ventilation and increased mortality in those with hyperactive platelets. In this study, changes in platelet activation were associated with increased platelet expression of P-selectin and CD63 in patients with more severe disease in comparison with those with mild manifestations. Platelets from patients with severe COVID-19 infection induce monocyte-derived tissue factor (TF) expression that is diminished by pretreating COVID-19 patient platelets with an anti–P-selectin neutralizing antibody or the clinically approved anti-αIIb/β3 monoclonal antibody, abciximab. Together, these findings again demonstrate the role of the platelet in COVID-19, linking changes in platelet activation and platelet-dependent monocyte TF expression with disease severity and mortality. Linking platelet-mediated monocyte TF expression to unbridled coagulation and thrombosis remains to be determined.
Although both of these articles clearly demonstrate differences in platelets and platelet function in patients with COVID-19, a well-defined connection between platelet hyperactivity and the coagulopathy seen in COVID-19 patients has not been confirmed. Dedicated studies comparing platelet function testing in COVID-19 patients with and without thrombosis have not been performed. Because platelets are already known to be hyperreactive in the setting of inflammation, linking platelet abnormalities directly to thrombotic risk is key to understanding whether the platelet is merely a bystander in the inflammatory milieu or a key pathological regulator of thrombosis in COVID-19.
An understanding of the role of the platelet in COVID-19–associated thrombosis has clear clinical implications in considering antiplatelet therapies for treating COVID-19 patients. The addition of antiplatelet therapy to patient management was addressed in both articles. In the study by Manne et al, platelet hyperreactivity in COVID-19 ICU patients was reduced by pretreatment of the platelets with high doses of aspirin. In the Hottz et al study, platelet-targeting agents, including aspirin and clopidogrel, did not prevent platelet-induced TF in monocytes, yet anti–P-selectin neutralizing antibodies and integrin targeting through abciximab disrupted platelet signaling to monocytes. These findings would suggest that directed blockade of platelet P-selectin and/or other key platelet-activation pathways may interfere with the platelet hyperreactivity observed in COVID-19. Because a modest rate of bleeding and thrombocytopenia have also been reported in COVID-19 patients, overall hemostasis must be considered before instituting antiplatelet therapies.13 The work of Manne et al and Hottz et al sets the stage for clinical trials evaluating the efficacy of antiplatelet drugs in COVID-19–associated coagulopathy.
Conflict-of-interest disclosure: The author declares no competing financial interests.