Triggering factors of vaso-occlusion such as cold or stress are well known by physicians who care for patients with sickle cell disease (SCD). Surprisingly, however, the underlying pathophysiology has remained rather elusive so far. In this issue of Blood, Veluswamy et al have identified that thermal stimuli (both heat and cold) as well as pain and anxiety result in microvascular constriction in patients with SCD.1 Importantly, they show that, unlike in healthy people, repeated stimuli in patients with SCD result in progressively increasing vasoconstriction, thereby setting the stage for vaso-occlusion, the central pathophysiological lesions associated with SCD.
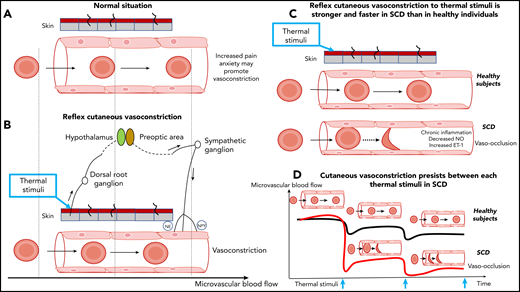
Reflex vasoconstriction to thermal stimuli in healthy individuals and patients with SCD. (A) Cutaneous vessel and flowing red blood cells at baseline in a healthy individual. (B) Normal reflex vasoconstriction to thermal stimuli applied to the skin and flowing red blood cells in a healthy individual. The x-axis at the bottom of the figure indicates red blood cell velocity. The release of norepinephrine (NE) and neuropeptide Y (NPY) causes vasoconstriction. The vertical dashed lines crossing panels A and B are arbitrary time points to show the impact of vasoconstriction on the red blood cell transit time. (C) Reflex vasoconstriction to thermal stimuli is faster and stronger in patients with SCD compared with healthy volunteers. Additional factors, such as inflammation, nitric oxide (NO) bioavailability, and endothelin-1 (ET-1) levels could modulate the level of this vasoconstrictive response. (D) The repetition of thermal stimuli results in progressive and increasing vasoconstriction in SCD patients whereas blood flow of healthy volunteers partially recovers. Vasoconstriction prolongs the transit time of red blood cells and increases the risk of vaso-occlusion. Blue arrows indicate thermal stimuli.
Reflex vasoconstriction to thermal stimuli in healthy individuals and patients with SCD. (A) Cutaneous vessel and flowing red blood cells at baseline in a healthy individual. (B) Normal reflex vasoconstriction to thermal stimuli applied to the skin and flowing red blood cells in a healthy individual. The x-axis at the bottom of the figure indicates red blood cell velocity. The release of norepinephrine (NE) and neuropeptide Y (NPY) causes vasoconstriction. The vertical dashed lines crossing panels A and B are arbitrary time points to show the impact of vasoconstriction on the red blood cell transit time. (C) Reflex vasoconstriction to thermal stimuli is faster and stronger in patients with SCD compared with healthy volunteers. Additional factors, such as inflammation, nitric oxide (NO) bioavailability, and endothelin-1 (ET-1) levels could modulate the level of this vasoconstrictive response. (D) The repetition of thermal stimuli results in progressive and increasing vasoconstriction in SCD patients whereas blood flow of healthy volunteers partially recovers. Vasoconstriction prolongs the transit time of red blood cells and increases the risk of vaso-occlusion. Blue arrows indicate thermal stimuli.
Vaso-occlusion pathophysiology is complex. It is currently viewed as a multistep process in which the time period required for sickle hemoglobin to polymerize is critical. If the time spent by deoxygenated red blood cells in microvessels is less than the time it takes for polymerization of deoxygenated sickle hemoglobin, the red blood cells flow to larger vessels before they sickle and entrapment is less likely to occur.2 Therefore, it requires additional mechanisms to precipitate subsequent vaso-occlusion. Adhesion of red or white blood cells to the endothelium, increased blood viscosity, or impaired vasodilation resulting from hemolysis-related nitric oxide (NO) depletion are some of the additional mechanisms that have been extensively studied.3,4 Veluswamy et al explore the contribution of microvascular vasoconstriction related to thermal stimuli or stress, which, within seconds, may reduce the microvessel diameter, decrease flow, and trap the red cells, whatever their state of sickling.
In an original experimental setup using healthy volunteers and SCD patients, Veluswamy et al explored microvascular blood flow responses to a sequence of hot and cold stimuli. Of note, the reduction of blood flow was measured within seconds on the hand opposite the hand that was receiving the stimulation. One important finding was that although hot/cold detection or pain thresholds did not differ between SCD patients and controls, there was a faster and stronger microvascular constriction to any given thermal stimulus in SCD patients and a cumulative decrease in perfusion with repeated thermal stimulation specific to SCD that did not recover within the experimental time frame (see figure). Although decreased microvascular blood flow does not necessarily result in vaso-occlusion, autonomic hyperresponsiveness to thermal stimulation or pain anxiety is an original observation in SCD that may help explain vaso-occlusion and offer new therapeutic targets.
SCD is characterized by a remarkable phenotypic heterogeneity that can only partly be explained by genetic factors.5 Environmental factors are thought to play an important role, but studies have shown conflicting results.6 Despite the empirical evidence of an increase in vaso-occlusive pain after exposure to cold, for instance, no clear association has been shown. The findings in the Veluswamy et al study potentially bridge the gap between triggering environmental factors and the occurrence of vaso-occlusive events. In patients prone to vasomotor hyperresponsiveness, environmental exposure to cold results in progressive decreased perfusion, and a small additional factor such as stress may be sufficient to further decrease regional perfusion and trigger vaso-occlusion. The reflex vasoconstriction response to stimuli involves neural-mediated mechanisms that ultimately lead to the release of norepinephrine and neuropeptide Y as vasoconstrictors.7 However, other key biological factors may also modulate the response to these thermal stimuli. The inability of microcirculatory vessels in SCD patients to recover to their baseline diameters between each stimulus could be partly linked to the magnitude of the decrease in NO bioavailability, which may vary over time, depending on the level of hemolysis. In addition, inflammation, which is chronically enhanced in SCD, could also cause dysfunction in sympathetic neurotransmitter regulatory mechanisms, particularly as a result of ageing.8 The combination of these factors may be found to modulate the sensitivity of microcirculatory vessels to external stimuli, resulting in various levels of vasoconstriction in SCD patients. The next step would be to study reflex vasoconstriction in larger cohorts of SCD patients and test the association with clinical severity, particularly the frequency of vaso-occlusive crises. Time to recovery of blood flow would be an interesting characteristic to be explored in the future. Likewise, how this parameter evolves with time, with repeated lesions or with treatment, would be exciting research areas.
This study should also foster further work on the effects of novel strategies to improve treatment for patients with SCD. Drugs for SCD have targeted hemoglobin F upregulation, adhesion, or hemoglobin-oxygen affinity to decrease severe vaso-occlusive complications.9 Decreasing or stabilizing the extent of vasoconstriction could represent a new therapeutic field. For instance, it has been shown that SCD patients with depression or anxiety had more vaso-occlusive crises and episodes of acute chest syndrome than patients with better mental quality of life.10 Increasing the use of cognitive therapy and antidepressant treatment strategies to relieve anxiety may improve the high vasoconstrictive phenotype of SCD patients and limit the risk of complications.
Overall, the article by Veluswamy et al uncovers a neglected aspect of SCD pathophysiology and paves the way to therapeutic options directed at stabilizing vasomotor reactivity that may help reduce disease severity.
Conflict-of-interest disclosure: The authors declare no competing financial interests.