In this issue of Blood, Su et al1 employ an elegant microRNA (miRNA or miR) conjugation strategy to unleash the potential of targeted miRNA therapeutics, and, as a proof of concept, effectively counteract common inflammatory and myeloid disease states.
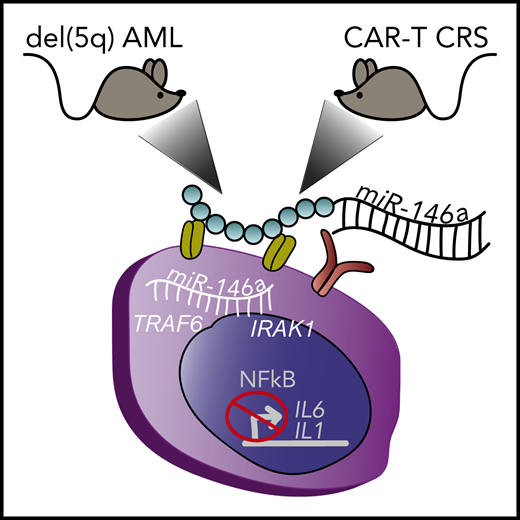
Depiction of 2 different mouse xenograft models of myeloid diseases treated with miR-146a conjugate therapy that enters myeloid cells through surface receptors. (Left) Mouse with human del(5q) AML. (Right) Mouse with human lymphoma that developed myeloid-derived CRS upon treatment with CD19 CAR-T cell therapy. Inside the myeloid cell, the delivered miR-146a targets TRAF6 and IRAK1, that block NF-κB–mediated production of inflammatory cytokines IL1 and IL6. del(5q), deletion of chromosome 5q.
Depiction of 2 different mouse xenograft models of myeloid diseases treated with miR-146a conjugate therapy that enters myeloid cells through surface receptors. (Left) Mouse with human del(5q) AML. (Right) Mouse with human lymphoma that developed myeloid-derived CRS upon treatment with CD19 CAR-T cell therapy. Inside the myeloid cell, the delivered miR-146a targets TRAF6 and IRAK1, that block NF-κB–mediated production of inflammatory cytokines IL1 and IL6. del(5q), deletion of chromosome 5q.
miRNA comprise a group of small noncoding RNA that negatively regulates target mRNA stability and/or translation in a sequence-directed manner to reduce target protein expression. miRNA are highly conserved across species and involved in a wide range of normal cellular and developmental processes. miRNA exhibit remarkable cell and tissue type specificity, such that perturbations in select miRNA expression directly contribute to disease states, including inflammation and cancer. miRNA expression is predictive and prognostic in hematologic malignancies, and circulating miRNA may serve as a biomarker of minimal residual disease.
A major goal in the field is to leverage miRNA for disease treatment by either blocking or restoring miRNA activity to normal levels, thereby relieving or imposing regulation on specific miRNA targets to counteract disease processes. Specific miRNAs with potential therapeutic benefit have been identified. A major obstacle is how to deliver miRNA-based therapies. Progress has been made in optimizing chemical modifications to nucleic acids that render RNA significantly more stable in vivo. However, bypassing liver uptake and metabolism and directing tissue or cell type specificity remain great challenges to miRNA or RNA interference–based therapeutics, particularly for hematologic diseases and malignancies.
Su et al focused their efforts on miR-146a as a therapeutic regulator to dampen NF-κB–mediated inflammatory signaling. miR-146a is a critical gene lost in del(5q) myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) that contributes to pathogenesis of these malignancies.2 Critical targets of miR-146a function within the Toll-like receptor (TLR) signaling pathway, including IRAK1 and TRAF6, which are important regulators of NF-κB transcription factor activity.3 The role of NF-κB in MDS and AML to date is largely context dependent; however, in the context of miR-146a loss, deregulation of IRAK1, TRAF6, and subsequent NF-κB activation leads to inflammatory cytokine production and myeloid malignancy.4,5 In addition, NF-κB–mediated production and release of interleukin-6 (IL6) from monocytes are important factors contributing to the cytokine release syndrome (CRS) associated with chimeric antigen receptor T-cell therapy (CAR-T) therapy in B-cell lymphoma.6,7 Remarkably, Su et al demonstrate that in vivo restoration of miR-146a expression, using a novel miR-146a conjugate discussed below, prolongs the survival of mice transplanted with human del(5q) AML cells and in lymphoma-bearing mice significantly reduces cytokine overproduction in response to CD19 CAR-T cell therapy (see figure). In vivo, miR-146a conjugate delivery significantly reduces the NF-κB signaling pathway and inflammatory cytokine production in both model systems. The significance of this work extends beyond the tested models because targeting the NF-κB pathway has potential therapeutic relevance in a variety of malignancies and diseases rooted by inflammation.
How were the in vivo challenges of uptake, specificity, and toxicity overcome in this study? Su and colleagues used a thoughtful miRNA conjugation strategy with impressive specificity for myeloid and, to a lesser extent B-cell, uptake. They selected a scavenger receptor/TLR9-targeting type A specific CpG oligodeoxynucleotide8 to conjugate to miR-146a. Critically, this type A oligodeoxynucleotide is specific for myeloid cells, but blunted in its ability to trigger a TLR9 immune response.9 These unique miRNA conjugate features set this study apart from other approaches.
In sum, miR-146a as a therapeutic to dampen inflammatory signaling is a novel strategy with an exciting future. This contribution by Su et al is a crucial step forward in the development of miRNA therapies targeting myeloid diseases and provides a foundation to build upon to further refine and innovate miRNA conjugate therapeutic strategies for other cell types. This conjugate method offers several advantages, including limited or no cytotoxicity in nonmyeloid cell types, myeloid targeting, and miRNA-mediated immunomodulation. As such, this is an attractive approach for other prospective miRNA or anti-miRNA therapeutics or as a mechanistic tool for dissection of miRNA function in myeloid biology.
Conflict-of-interest disclosure: S.E.M. declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal