In this issue of Blood, Tausch and colleagues demonstrated the clinical benefit of venetoclax and obinatuzumab for IGHV-unmutated chronic lymphocytic leukemia (CLL) and for all other CLL genetic subgroups with the exception of del(17p) and mutated TP53, within a phase 3 trial.1
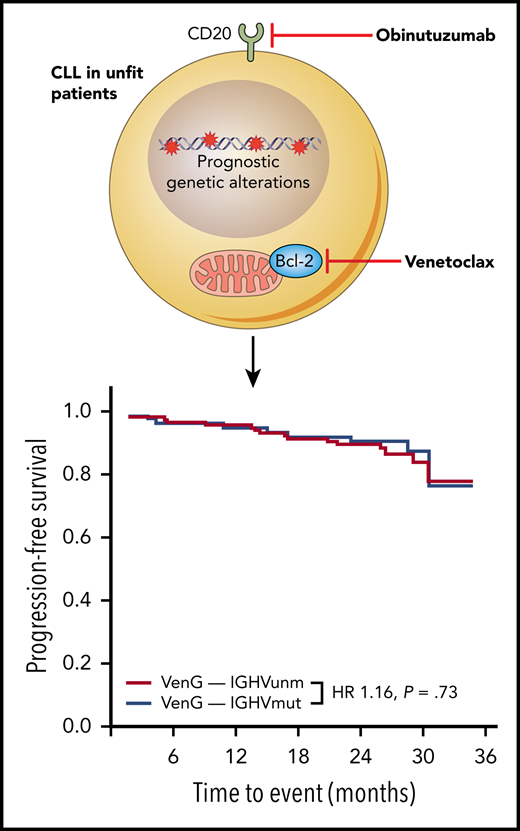
A novel combination of an anti-CD20 antibody obinatuzumab (G) and the Bcl-2 inhibitor venetoclax (Ven) provides a new solution for unfit CLL patients. This combination resulted in durable remissions on the CLL14 trial regardless of IGHV mutational status, therefore overcoming unmutated VH status as an indicator of poor prognosis. HR, hazard ratio; IGHVmut, IGHV mutated; IGHVunm, IGHV unmutated.
A novel combination of an anti-CD20 antibody obinatuzumab (G) and the Bcl-2 inhibitor venetoclax (Ven) provides a new solution for unfit CLL patients. This combination resulted in durable remissions on the CLL14 trial regardless of IGHV mutational status, therefore overcoming unmutated VH status as an indicator of poor prognosis. HR, hazard ratio; IGHVmut, IGHV mutated; IGHVunm, IGHV unmutated.
We are heading into a new era in our battle against CLL. Knowledge of CLL biology acquired from basic science research has led to transformative treatments that are revolutionizing the CLL therapeutic landscape. Chemoimmunotherapy, which until a few years ago was the mainstay of CLL treatment, is increasingly being replaced by chemo-free treatment options. Chemoimmunotherapy is effective in a proportion of CLL patients but much less so among those with high-risk genetic features, including deletions of 17p or 11q and TP53, ATM, SF3B1, and NOTCH1 mutations, as well as unmutated IGHV. In these patients, small molecule inhibitors targeting B-cell receptor signaling (eg, ibrutinib) and the antiapoptotic protein Bcl-2 (eg, venetoclax) are producing response rates that were unimaginable with previous chemoimmunotherapy, and often with less toxicity. It remains unclear, however, whether these excellent treatment responses translate into long-term, durable remissions for all CLL genetic subgroups and whether genetic stratification in this new era of CLL targeted therapies still matters.
In this important study, Tausch and colleagues have provided us with some answers to these pertinent questions. Here, the clinical impact of genetics is studied in the context of the CLL14 trial, which establishes the combination between immunotherapy, anti-CD20 antibody, obinatuzumab, and the Bcl-2 inhibitor, venetoclax, as a new standard of care for previously untreated, elderly, and less fit CLL patients with comorbidities.2 In contrast to chemoimmunotherapy, where unmutated IGHV and adverse genetics are predictive for unfavorable outcome, durable remissions were observed with this combination regardless of IGHV mutational status (see figure), del(11q), trisomy 12, or mutations of ATM, SF3B1, BIRC3, and NOTCH1. Similar observations have been reported with ibrutinib within the RESONATE and RESONATE-2 trials with the exception that in the RESONATE study patients with SF3B1 mutations showed a trend toward inferior progression-free survival.3,4
Tausch and colleagues also observed high rates of response and minimal residual disease negativity in patients with del(17p) and/or TP53-mutant CLL following combined venetoclax/obinatuzumab treatment, but these responses were often not durable. Shorter progression-free survival has also been reported in TP53-defective CLL treated with venetoclax and rituximab within the MURANO study,5 and with B-cell receptor signaling inhibitors.3,6 The p53 protein mediates apoptosis in response to DNA damaging agents but the loss of p53 dependent apoptosis cannot explain the limited anti-CLL effects of targeted therapies. One potential explanation is that TP53-defective CLL cells are more genomically unstable owing to the pivotal role p53 plays in the DNA damage response during DNA replication. Indeed, clonal evolution and genomic complexity frequently precede disease progression in patients treated with venetoclax or ibrutinib.7-9
Venetoclax-based therapies remain expensive. The greatest clinical significance of the current study is that it helps us define CLL treatments according to genetic subgroups. In patients with IGHV-mutated CLL with no genetic aberrations other than del(13q), there may be continued justification for the use of chemoimmunotherapy providing there are no significant tolerability issues. Patients with IGHV-unmutated CLL and those with del(11q), trisomy 12, ATM, NOTCH1, SF3B1, and BIRC3 mutations are the individuals who stand to benefit the most from venetoclax with obinatuzumab over chemoimmunotherapy. Finally, patients with del(17p) and TP53 mutations also derive some benefit from venetoclax/obinatuzumab compared with chemoimmunotherapy, but should ideally be enrolled in clinical trials exploring novel therapies and therapeutic combinations.
Finding the best way to manage TP53-defective CLL undoubtedly continues to be a pressing task. It remains to be to determined whether prolonged venetoclax treatment and a higher depth of response could prevent disease relapse and whether the use of ibrutinib and venetoclax in combination could overcome resistance to either agent. It will also be of interest to establish how effective chimeric antigen receptor T cells and other immunotherapies are and whether better understanding of the subclonal dynamics, resistance mechanisms, and the pathways on which TP53-defective CLL rely for their survival can improve clinical outcomes in this genomically unstable CLL subgroup.
Over the past decade, treatments have been developed that are highly successful in targeting pathogenic processes common to all CLL cells. To secure durable disease control, or even a cure, future research efforts will need to take the therapeutic vulnerabilities of resistant CLL subclones into consideration. Clearly, the arrival of targeted therapies has not consigned CLL genetics to irrelevance. Quite the opposite, Tausch et al have shown us that genetics continues to influence treatment outcome, and provides us with important pointers for future research.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal