In this issue of Blood, Al-Sawaf et al1 show that a complex karyotype (CKT, defined as 3 or more chromosomal abnormalities in 2 or more metaphases) has predictive value in treatment-naive patients with chronic lymphocytic leukemia (CLL) who received chlorambucil-obinutuzumab (ClbG) in the randomized CLL14 trial,2 whereas in patients randomized to receive venetoclax-obinutuzumab (VenG), the CKT did not impact the clinical outcome.
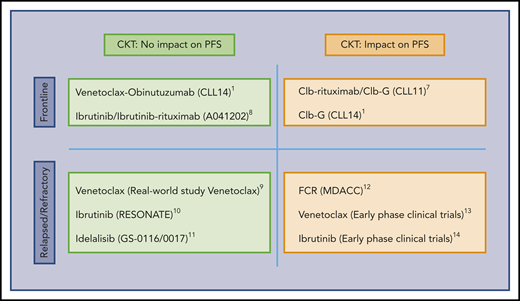
Influence of CKT on the PFS of CLL patients. The predictive value of CKT in CLL treated with target therapy remains to be established. CKT is associated with shorter PFS in patients treated with chemoimmunotherapy combinations and in many studies of heavily pretreated patients included in early-phase clinical trials. In contrast, the current study suggests that activity of some targeted therapies employed at initial treatment may not be influenced by the presence of CKT. Clb, chlorambucil; FCR, fludarabine, cyclophosphamide, rituximab; G, obinutuzumab.1,7-14
Influence of CKT on the PFS of CLL patients. The predictive value of CKT in CLL treated with target therapy remains to be established. CKT is associated with shorter PFS in patients treated with chemoimmunotherapy combinations and in many studies of heavily pretreated patients included in early-phase clinical trials. In contrast, the current study suggests that activity of some targeted therapies employed at initial treatment may not be influenced by the presence of CKT. Clb, chlorambucil; FCR, fludarabine, cyclophosphamide, rituximab; G, obinutuzumab.1,7-14
Over the past 7 years, therapeutic paradigms for patients with CLL have changed based on the use of clinically validated targeted therapies.3 However, it is important to evaluate the results of these therapies in known risk groups in CLL, such as those with a CKT.
Expression of the BCL-2 family protein members that tightly regulate the apoptotic process is skewed toward a phenotype aimed to evade apoptosis in CLL.4 Blocking BCL-2 protein function by the BH3 mimetic drug venetoclax leads to apoptosis of CLL cells and disease control.5 Recently, VenG was shown to be more active than ClbG in the CLL14 trial. This trial examined initial treatment of CLL patients with comorbidities defined by the presence of a CIRS (Cumulative Illness Rating Scale) >6 and/or creatinine clearance <70 mL/min.2 Why was this study by Al-Sawaf et al performed? Biological and genetic analyses in most of the trials employing targeted therapies are somewhat limited, making analysis of response in patients with previously identified biomarkers difficult. Among these biomarkers, CKT, defined by the presence of ≥3 chromosomal aberrations, has been proved to be an excellent predictor for progression-free survival (PFS) in patients treated with chemoimmunotherapy, in particular, when ≥5 aberrations are present or in the relapsed/refractory setting.6,7 The prognostic impact of CKT in patients receiving the newer, targeted treatments has not been established (see figure). The authors analyzed the impact of the presence of CKT in patients in the CLL14 trial.2 What are the lessons learned from this study? In this study, CKT was present in 30 patients (15.2%) in the ClbG arm and in 34 patients (17.0%) in the VenG arm, with similar clinical and biological characteristics in the patients in both arms. As expected, patients with CKT in the ClbG arm had a lower overall response (ORR; 50% vs 78%), complete response (CR; 10% vs 27.5%), and undetectable minimal residual disease (uMRD; <10−4; 20% vs 40.1%) rates, and a shorter PFS at 2 years (36.6% vs 69.6%) and overall survival (OS) at 2 years (82.7% vs 95.1%) than patients without CKT in the same arm. Remarkably, however, the presence of CKT did not interfere with ORR, CR, uMRD rates, or PFS and OS outcomes in patients in the VenG arm. This targeted combination was able to vanquish the deleterious effect of the CKT. This appears to agree with other studies, where targeted therapies in non–heavily pretreated patients can overcome the negative impact of CKT (see figure).
This study has significant limitations, including the small number of patients and short follow-up, and that the specific value of recurrent genetic lesions in CLL is not analyzed or combined with chromosomal aberrations. This study does highlight the need for revisiting the impact of predictive and prognostic factors in the post–chemoimmunotherapy era. Regrettably, one of the drawbacks of the use of conventional karyotypes is that this methodology does not weigh the occurrence of known high-risk chromosome aberrations as greater than others or the existence of specific genetic lesions with predictive value in identifying patients who would benefit from a particular treatment. In an era where medicine points toward precise therapeutic strategies based on specific genetic and biological targets, CKT still provides a rough analysis of the genome complexity. In summary, frontline VenG is an active combination in patients with CLL and comorbidities who present with CKT. Whether this parameter should be incorporated in the standard workout of patients with CLL deserves further validation.
Conflict-of-interest disclosure: F.B. has received research funding and honoraria from Roche, Celgene, Takeda, AstraZeneca, Novartis, Abbie, Kite, Kariospharm, Sanofi, and Janssen. P.A. has received honoraria from Janssen, Roche, Celgene, and Abbie.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal