In this issue of Blood, Liu et al describe the favorable response of adults with relapsed/refractory Epstein-Barr virus (EBV)-associated hemophagocytic lymphohistiocytosis (HLH) to treatment with nivolumab, a programmed cell death-1 (PD-1) inhibitor.1 EBV-HLH presents a challenging clinical conundrum because only a minority of patients will achieve long-standing clinical remission with front-line therapy.2 To further complicate matters, neither clinical nor pathologic-based criteria have been well established to differentiate which patients are likely to fail upfront HLH therapy with etoposide and dexamethasone.3,4 Patients with relapsed/refractory disease have a dismal chance of survival because of high rates of disease-related mortality.2,3
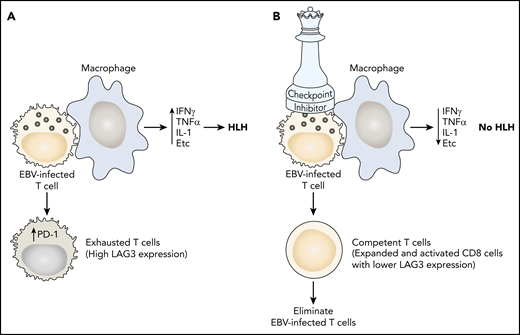
(A) In EBV-HLH, the viral infection causes a hyperinflammatory interaction with T cells and macrophages, resulting in excessive production of interferon-γ (IFNγ), tumor necrosis factor α (TNFα), interleukin-1 (IL-1), and other cytokines leading to HLH. Increased expression of PD-1 renders cytotoxic T cells incapable of controlling the infection (exhaustion). (B) Nivolumab binds to PD-1, rendering T cells competent to control the EBV infection and extinguish HLH.
(A) In EBV-HLH, the viral infection causes a hyperinflammatory interaction with T cells and macrophages, resulting in excessive production of interferon-γ (IFNγ), tumor necrosis factor α (TNFα), interleukin-1 (IL-1), and other cytokines leading to HLH. Increased expression of PD-1 renders cytotoxic T cells incapable of controlling the infection (exhaustion). (B) Nivolumab binds to PD-1, rendering T cells competent to control the EBV infection and extinguish HLH.
Various salvage regimens have been used for relapsed/refractory EBV-HLH, including combination chemotherapy regimens, monoclonal antibodies targeting the host cellular reservoirs for EBV, and targeted agents aimed at controlling the systemic inflammatory syndrome that defines the disease pathophysiology.3 Nivolumab presents a novel approach to EBV-HLH as it seeks to restore T-cell immune function against uncontrolled EBV infection, which is at the very root of this virally mediated disease process. Because immune checkpoint inhibition of PD-1 has proven a useful therapeutic option for relapsed/refractory EBV-related lymphomas, it offers an attractive novel option for EBV-HLH as well.5 Although it is generally accepted that relapsed/refractory EBV-HLH will ultimately require allogeneic hematopoietic stem cell transplant (HSCT) to achieve a cure, the authors sought to explore whether restoration of immune function through PD-1 inhibition could lead to long-standing control of EBV infection and the associated HLH syndrome.3
In this case series of 7 adults with relapsed/refractory EBV-HLH, nivolumab monotherapy resulted in clinical complete remission in 5 patients with a median follow-up of 16 months. The clinical successes were corroborated by translational experiments using single-cell transcriptome analyses. These demonstrated baseline overexpression of inflammatory markers, including tumor necrosis factor, interleukin-1B, and CD163. They also demonstrated expansion of PD-1+ T cells after treatment with nivolumab, which was associated with decreasing levels of interferon-γ and granzyme B (cytokines that drive the hyperinflammatory syndrome characteristic of HLH), enrichment of CD8+ T cells in activation and degranulation pathways, and a correlative decrease in the EBV viral loads in 4 of the 5 patients who achieved clinical remission. Single-cell RNAseq analyses of CD8+ T cells at baseline revealed underexpression of specific HLH-related genes, including STXBP2, UNC13, SH2D1A, and CD27, suggesting that such immune dysregulation may explain the vulnerability to EBV-related complications. Thus, immune checkpoint inhibition with nivolumab effectively restored T-cell immune competence against EBV, resulting in clinical improvement of the associated HLH (see figure).
Although immune checkpoint inhibition has recently garnered widespread excitement for its role in anticancer therapy, elegant in vivo experiments demonstrating the potential immunological role in the treatment of viral infections was reported 14 years ago.6 These studies showed that PD-1 expression was upregulated in exhausted T cells, and that PD-1 inhibition helped restore the dysfunctional CD8 T-cell immune response against viral infection.6 More recent work has highlighted the impact of T-cell exhaustion in other infections (eg, malaria, HIV, hepatitis B virus) and the role of immune checkpoint blockade in treating infectious diseases as well.7 In addition, immune checkpoint blockade has been evaluated in patients with virus-associated malignancies, in particular, in patients living with HIV and cancer, and has generated encouraging results leading to several ongoing prospective clinical trials.8
This case series of adults with EBV-HLH treated with nivolumab offers a glimmer of hope for improving the treatment of EBV-HLH, particularly for those patients with relapsed/refractory disease. Although the authors did not expand their discussion to the closely related spectrum of diagnoses that fall under the category of systemic EBV-associated T- and natural killer (NK)-cell lymphoproliferative diseases, there may be some potential overlap in the utility of PD-1 inhibition for this extremely challenging subset of diseases.
The updated World Health Organization classification of lymphoid neoplasms has categorized systemic EBV-associated T- and NK-cell lymphoproliferative diseases as a closely related group of diagnoses with extensive clinical and pathological overlap. Included in this umbrella of diagnoses are EBV-HLH, systemic T- and NK-cell chronic active EBV, and the systemic EBV-positive T-cell lymphoma of childhood.9,10 Patients who have HLH and EBV viremia may follow one of the following clinical scenarios: (1) HLH secondary to EBV infection that is effectively cured with front-line HLH therapy, (2) HLH driven by EBV infection in the context of an underlying genetic predisposition to EBV-associated lymphoproliferative disease that requires allogeneic HSCT for cure, (3) HLH driven by EBV as the clinical manifestation of what eventually is diagnosed as T- or NK-cell chronic active EBV, (4) HLH driven by EBV as the clinical manifestation of the systemic EBV-positive T-cell lymphoma of childhood.9,10 The latter 2 diagnoses are associated with extremely high rates of disease-related mortality and are only cured through allogeneic HSCT.9,10 This group of EBV-associated T- and NK-cell lymphoproliferative diseases are in desperate need of novel therapeutic approaches to offer hope for improved outcomes.
The work of Liu et al presents exciting preliminary data on the potential role of immune checkpoint inhibition for the treatment of EBV-HLH. Although it must be validated with larger, prospective cohorts of patients with longer off-therapy follow-up, it offers the possibility that anti-PD-1–targeted therapy may restore immune function against a disease mediated by EBV infection. It might also offer a potential therapeutic avenue for the treatment of systemic T- and NK-cell chronic active EBV and the systemic EBV-positive T-cell lymphoma of childhood, disease processes that are closely related to EBV-HLH.
Conflict-of-interest disclosure: K.L.M. is on the medical advisory board for Sobi Corporation. N.K.E.-M. declares no competing financial interests.