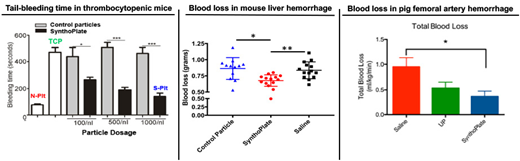
Platelets are primarily responsible for staunching bleeding by forming a 'platelet plug' and further amplifying thrombin generation on its surface to facilitate fibrin formation, leading to hemostatic clot formation at the site of vascular breach. Therefore, platelet transfusions are clinically used to mitigate bleeding risks in thrombocytopenia (prophylactic transfusion) and to mitigate hemorrhage in traumatic injuries (emergency transfusion). Currently these transfusions utilize donor-derived platelets, stored at 20-24oC with gentle agitation. In this condition, platelets have high risk of bacterial contamination and very short shelf-life (~ 5 days), which severely limit their logistical availability and use. Several parallel strategies are currently undergoing research to address these issues, including platelet storage at reduced temperatures (chilled or freeze-dried), pathogen reduction technologies and bioreactor-based in vitro platelet production from precursor cells. An alternative (and complimentary) approach that is the focus of our research is the engineering of I.V.-administrable synthetic hemostat nanoparticles that functionally mimic platelet's clotting mechanisms. These 'synthetic platelet' nanoparticle systems can be manufactured at large scale, sterilized without compromising functions and stored for long periods of time (6-9 months), thereby allowing significant logistical advantages in transfusion applications. Here we present in vitro and in vivo evaluation of such technology. For these studies, the 'synthetic platelet' nanoparticles were manufactured by decorating liposomes with a combination of VWF-binding, collagen-binding and fibrinogen-mimetic peptides, for integrative mimicry of platelet's hemostasis-relevant adhesive and aggregatory mechanisms. The nanoparticles were stored at room temperature in aqueous suspension as well as lyophilized powder, and particle stability was assessed over 6-9 months by dynamic light scattering (DLS). The nanoparticles were also exposed to E-beam sterilization, and particle stability as well platelet-mimetic bioactivity was assessed by DLS, aggregometry, microfluidics and rotational thromboelastometry (ROTEM). The systemic safety and targeted hemostatic efficacy of I.V.-administered nanoparticles were evaluated in mouse model of thrombocytopenia, and in mouse, rat and pig models of traumatic hemorrhage. DLS and electron microscopy confirmed that the synthetic platelet nanoparticles have a size of 150-200 nm diameter, and they remain stable over 6-9 months in storage. Microfluidic studies showed that these nanoparticles could rapidly adhere to 'vWF + collagen'-coated surfaces and enhance the recruitment and aggregation of active platelets on these surfaces. Aggregometry studies showed that the nanoparticles did not affect resting platelets but enhanced aggregation of ADP- or collagen-activated platelets (i.e. no thrombotic risk towards resting platelets). Flow cytometry studies confirmed this specificity of nanoparticle binding to active platelets. ROTEM studies showed that the 'synthetic platelet' nanoparticles significantly improved clot kinetics and firmness. In vivo, in all animal models, the nanoparticles showed no systemic pro-thrombotic effects, as assessed by hemodynamics as well as organ histology. In thrombocytopenic mice, prophylactically administered 'synthetic platelet' nanoparticles dose-dependently reduced tail bleeding time. In mouse, rat and pig trauma models, post-injury administration of 'synthetic platelet' nanoparticles reduced blood loss, stabilized blood pressure, delayed hypotension and thereby significantly improved survival. The nanoparticles could be further utilized as a platform for targeted presentation of phosphatidylserine (PS) to augment thrombin generation, or targeted delivery of tranexamic acid (TXA) for anti-fibrinolytic effect or delivery of inorganic polyphosphate (PolyP) to augment clot stability. These studies not only establish the potential of these nanoparticles as a platelet surrogate for transfusion applications, but also demonstrate their utilization as a platform for modular augmentation of various hemostatic outputs in prophylactic and emergency applications.
Sen Gupta:Haima Therapeutics LLC: Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal