
Introduction:
Iron deficiency anemia (IDA) is the most common cause of anemia worldwide and is frequently untreated or undertreated (Kesselbaum et al. 2014). The association of thrombocytosis with IDA is well-recognized, with recent work elucidating its underlying physiology (Xavier-Ferrucio et al. 2018). Thrombocytosis in IDA may predispose to thrombotic complications, as described in many case reports. Given that IDA is ubiquitous, even minor increases in thrombotic risk due to thrombocytosis have dramatic public health implications. Data describing the rate, predictors, and risk of thrombotic complications are limited. This study characterizes these features of thrombocytosis in IDA.
Methods:
We queried a large institutional patient data repository containing comprehensive chart data for over 5.8 million patients to identify patients with IDA with and without thrombocytosis and thrombotic events over a 40-year time period (1979 to 2019). Demographic information, hematological parameters, thrombosis history, and other medical history were collected at time of peak thrombocytosis, time of IDA diagnosis, and clinical baseline (before IDA diagnosis or after resolution). The fidelity of query data was assessed by performing detailed manual chart review of 700 total patients. Clinical diagnoses of IDA were verified by confirming ferritin values <30 ng/ml and consistent CBC parameters. Temporal association of thrombocytosis with IDA was confirmed and patients with other causes of thrombocytosis (including active malignancy, infection, inflammatory bowel disease, recent surgery/trauma, inflammatory arthritis, and myeloproliferative neoplasms) were excluded. Only arterial and venous thrombotic events occurring concurrently with IDA or IDA with thrombocytosis were included. Data were analyzed using t-tests, chi-square tests, Pearson correlations, and multivariate regression.
Results:
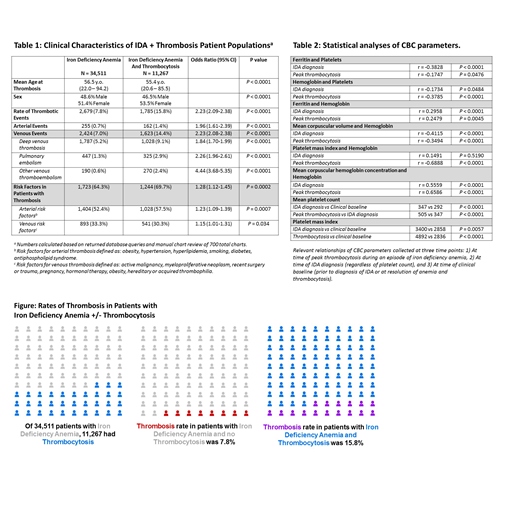
Our queries identified 36,327 IDA cases and 15,022 IDA with thrombocytosis cases. Query fidelity rates were 95% and 75%, respectively, yielding a true thrombocytosis rate of 32.6% in patients with IDA. After excluding cases with other causes of thrombocytosis, we calculated a 7.8% rate of thrombosis in patients with IDA and a 15.8% rate of thrombosis in patients with reactive thrombocytosis secondary to IDA (Table 1, Figure). The odds ratio for thrombosis in IDA with thrombocytosis vs IDA alone was 2.23 (2.09-2.38, p<0.0001). Both venous and arterial thrombotic events were more likely in patients with IDA and thrombocytosis (Table 1). Risk factors for thrombotic events were slightly more common in patients with IDA and thrombosis (OR 1.28, Table 1). Mean duration of IDA episode (from IDA diagnosis to normalization of hemoglobin and platelet count) was 2.03 years.
Among patients with IDA and thrombocytosis, ferritin and hemoglobin (Hgb) were both moderately negatively correlated with platelet count (Plt) (Table 2). Platelet mass index (Plt x MPV) at time of IDA diagnosis and peak thrombocytosis was significantly higher than baseline. Platelet mass index and Hgb were strongly negatively correlated at peak thrombocytosis. Multivariate regression including age, sex, WBC count, Hgb, Plt, and MCV demonstrated a significant predictive relation between decreasing Hgb and increasing Plt at peak thrombocytosis.
Conclusions:
In this large retrospective analysis, we describe the rate and predictors of thrombocytosis in IDA in addition to rates of thrombosis in IDA patients with and without reactive thrombocytosis. Combining queries of a massive clinical database with manual chart review to ensure data validity, we found a 32.6% rate of thrombocytosis in IDA, negative correlations between both Hgb/ferritin and Plt, and a 2-fold increased thrombotic risk in patients with thrombocytosis reactive to IDA relative to IDA alone. We also found increased total body platelet mass in IDA patients with thrombocytosis, which may contribute to this increased thrombotic risk. Thrombosis rates overall were high, possibly due to database inclusion of more morbid inpatients in addition to outpatients. Given the global burden of untreated and undertreated IDA, our findings suggest that adequate IDA treatment may reduce thrombotic complications and associated morbidity and mortality.
Acknowledgements:
A. Song is the recipient of the American Society of Hematology HONORS Award (provides research support).
Kuter:Daiichi Sankyo: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Merck Sharp Dohme: Consultancy, Honoraria; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Caremark: Consultancy, Honoraria; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Platelet Disorder Support Association: Consultancy, Honoraria; Alnylam: Consultancy, Honoraria, Research Funding; Zafgen: Consultancy, Honoraria; UCB: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Kezar: Research Funding; Principia: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Kyowa-Kirin: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Dova: Consultancy, Honoraria; Protalix: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Shinogi: Consultancy, Honoraria; Rigel: Consultancy, Honoraria, Research Funding. Al-Samkari:Agios: Consultancy, Research Funding; Dova: Consultancy, Research Funding; Moderna: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal