Introduction
The United States Thrombotic Microangiopathy (USTMA) Consortium consists of high-volume US referral centers that are committed to collaborative research in TMAs. The USTMA Immune Thrombotic Thrombocytopenic Purpura (iTTP) registry has compiled retrospective data on demographics, treatments and outcomes in patients with iTTP to create the world's largest database of patients with this rare disease.
While there is consensus on the use of therapeutic plasma exchange (TPE) for treatment of iTTP, there are no large randomized trials on which to base use of rituximab. The drug is frequently used for refractory or relapsed iTTP, but is currently being used more frequently for de novo (first episode) iTTP. We queried the USTMA iTTP registry to determine whether relapse free survival (RFS) is superior when rituximab is added to TPE and corticosteroids for treatment of iTTP. We hypothesized that the addition of rituximab would improve RFS at 5 years in both de novo and relapsing iTTP.
Methods
Following IRB approval at each institution, investigators independently reviewed individual patient records to confirm diagnostic criteria and entered demographic, treatment and outcomes data into the REDCap database housed at the University of North Carolina. The diagnosis of iTTP was defined as ADAMTS13 < 10% or ADAMTS13 < 20% with an inhibitor or antibody detected at any point or a clinical diagnosis of iTTP based on presenting characteristics, response to treatment and/or relapsing phenotype before ADAMTS13 testing became available (N=173). Relapse was defined as a recurrence of iTTP after at least 30 days of remission (recurrence within 30 days was considered an exacerbation, or continuation of the prior episode).
To explore the effect of rituximab added to TPE and corticosteroids, we first assessed the treatment effect in de novo iTTP patients and then separately in relapse. We constructed Kaplan-Meier curves to compare RFS for patients treated with rituximab plus corticosteroids versus corticosteroids alone in both groups, and compared RFS at specific time points using the Klein method. To better understand whether other patient variables had an effect on RFS in both de novo episodes and relapses, ordinary (time-to-event) and mixed-effects (recurrent time-to-event) Cox proportional hazards models were used to examine the relationships of treatment, race/ethnicity, sex, age, treatment year, and presenting signs/symptoms with the outcome. Analyses were conducted using R version 3.5.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
As of July, 2019, the USTMA database contains 775 unique study patients with a confirmed diagnosis of iTTP with 1397 unique iTTP episodes. The treatment of patients' de novo iTTP episode was available for analysis in 375 patients, 188 of whom were treated with corticosteroids alone, 131 with corticosteroids plus rituximab, and 56 with other therapies. RFS was significantly higher in patients treated with corticosteroids and rituximab compared to those treated with corticosteroids alone at 1 year (0.93 vs. 0.78, p=0.0002) and 3 years (0.82 vs. 0.66, p=0.004) but not 5 years (0.60 vs. 0.56, p=0.39). In addition, the risk of relapse decreased with later treatment year for de novo iTTP (hazard ratio (HR) 0.95, 95% CI 0.92-0.99, p=0.03), consistent with rituximab use increasing over time, and was increased in African Americans compared with Caucasians (HR 1.83, 1.10-3.06, p=0.02).
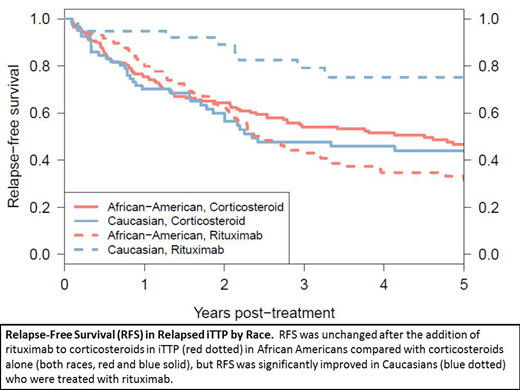
We then explored the treatment effect in all iTTP relapses (743 relapses in 426 patients). Here, a significant (p=0.0007) interaction between treatment and race was found. Among African Americans, we found no difference in RFS when rituximab was added (HR 1.15, 0.81-1.62, p=0.43). However, among Caucasians, RFS was significantly improved when rituximab was added (HR 0.15, 0.06-0.35, p<0.0001).
Conclusions
For de novo iTTP, adding rituximab to corticosteroids for immunosuppression likely delays but does not prevent relapse. Unlike in de novo disease, in patients with relapsed iTTP, we found a novel and significant interaction between race and treatment: while Caucasians had significantly improved RFS with the addition of rituximab, there was no effect on RFS in African Americans. Further investigation is warranted to determine the mechanisms of this difference in the response to rituximab in relapsed iTTP to improve outcomes in African Americans.
Marshall:Sanofi: Membership on an entity's Board of Directors or advisory committees. Farland:Sanofi: Membership on an entity's Board of Directors or advisory committees. Metjian:Sanofi: Membership on an entity's Board of Directors or advisory committees. Raval:Bayer, Inc: Research Funding; Sanofi: Membership on an entity's Board of Directors or advisory committees. Liles:Imara: Other: PI on Clinical trial- Sickle cell ; Shire: Other: PI on clinical trial Sickle cell ; Novartis: Other: PI on clinical trial Sickle cell . Baumann Kreuziger:CSL Behring: Consultancy; Vaccine Injury Compensation Program: Consultancy. McCrae:Rigel Pharmaceutical: Membership on an entity's Board of Directors or advisory committees; Sanofi Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Dova Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Pfizer Pharmaceutical: Membership on an entity's Board of Directors or advisory committees. Chaturvedi:Shire/Takeda: Research Funding; Sanofi: Consultancy; Alexion: Consultancy. Zheng:Clotsolution: Other: Co-Founder; Shire/Takeda: Research Funding; Ablynx/Sanofi: Consultancy, Speakers Bureau; Alexion: Speakers Bureau. Cataland:Ablynx/Sanofi: Consultancy, Research Funding; Alexion: Consultancy, Research Funding.
rituximab for immunosuppression in TTP.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal