Introduction: Our objective was to develop a prognostic model that predicts progression-free survival (PFS) and overall survival (OS) to enable risk-adapted strategies in patients with previously untreated diffuse large B-cell lymphoma (DLBCL). We retrospectively investigated the value of quantitative image texture features (i.e. 'radiomics' evaluating tumor heterogeneity) using FDG PET/CT data sets in a large, prospective Phase III trial, GOYA (NCT01287741).
Methods: In the GOYA trial, which compared obinutuzumab versus rituximab both in combination with CHOP chemotherapy, there was no significant treatment effect between the two arms, thus the two arms were combined for this study. Baseline PET/CT images with regions of interests (ROIs) defined by qualified physicians were analyzed for radiomics features. Image texture features (ITF) were computed using the open-source and validated PET Oncology Radiomics Test Suite (PORTS). The clinical risk factors (International Prognostic Index [IPI], Ann Arbor stage, extranodal disease, bulky disease), cell of origin (COO), standard PET-derived metrics (standard uptake value [SUV]-mean, SUV-max, total metabolic tumor volume [TMTV], total lesion glycolysis [TLG]), SUV histogram metrics (variance, skewness, and kurtosis), and ITF were evaluated for prediction of PFS and OS. TMTV was estimated using adaptive thresholding. Prognostic models were generated by means of multivariate Cox regression analysis, modeling PFS, and OS. In the absence of an independent patient cohort for external model validation, an internal validation, based on c-index and Brier score, was carried out using bootstrap resampling methods. Stratification of patients into risk groups was achieved through maximally selected rank statistics. Multivariate analysis was also carried out on a subgroup of patients with available COO information.
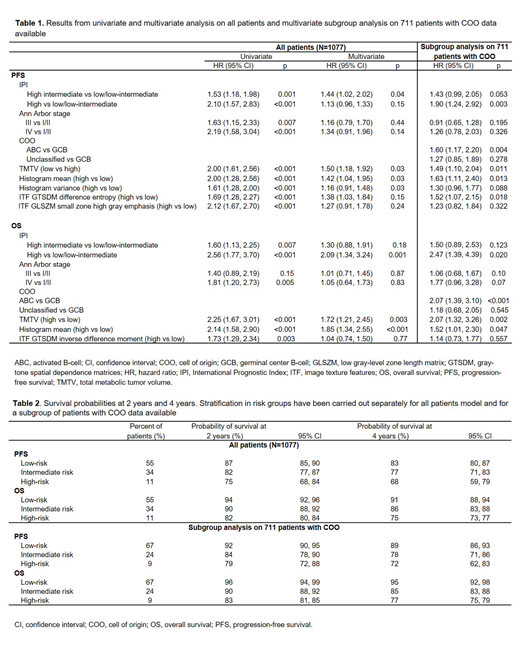
Results: The median follow-ups for PFS and OS were 46 and 50 months, respectively. Baseline PET scans were available for 1334 patients with detectable lesions, and 1077 baseline scans were evaluable for calculating ITFs. In the univariate analysis, high TMTV, histogram mean, histogram variance, and the ITFs gray-tone spatial dependence matrices (GTSDM) difference entropy and low gray-level zone length matrix (GLSZM) small zone high gray emphasis were risk factors for PFS, while high TMTV, histogram mean, and the ITF GTSDM inverse difference moment were risk factors for OS (Table 1, showing 95% CI, HR, and p-values for both univariate and multivariate analyses). In multivariate analysis, the risk factors included IPI, Ann Arbor stage, high TMTV, histogram mean, and GTSDM inverse difference moment; results were generally consistent in the multivariate subgroup analysis on patients with COO data available (Table 1). Based on the multivariate model, the probabilities for PFS and OS at 2 and 4 years for individual patients were established (Table 2). By combining TMTV (four categorical groups) with ITF, COO, and predictive clinical factors, three prognostic subgroups of treatment failure risk were identified: low (55% of patients), intermediate (34%), and high (11%). Hazard ratios for high and intermediate risk compared with low risk were 2.16 (p<0.001) and 1.17 (p=0.004) for PFS, and 3.82 (p<0.001) and 1.85 (p<0.001) for OS. The corresponding probability of survival at 2-years for high, intermediate and low risk groups were 87%, 82%, and 75% for PFS, and 94%, 90%, and 82% for OS. The 4-year survival probabilities were 83%, 77%, and 68% for PFS, and 91%, 86%, and 75% for OS (Table 2). For PFS, the accuracy of the Cox model was 0.63 with clinical variables only, 0.65 with the addition of TMTV, and 0.69 with the addition of ITFs; for OS, the corresponding values were 0.63, 0.65, and 0.70.
Conclusion: A model including PET-derived quantitative ITF, in addition to significant clinical features, was able to predict survival probability for untreated DLBCL patients with good precision. The proposed PET-based prognostic model may help identify patients who could benefit from risk-adapted treatment modifications or novel approaches.
Acknowledgments: GOYA was sponsored by F. Hoffmann-La Roche Ltd. Third-party editorial assistance, under the direction of Lale Kostakoglu, was provided by Katie Smith of Gardiner-Caldwell Communications and was funded by F. Hoffmann-La Roche Ltd.
Kostakoglu:F. Hoffman-La Roche: Consultancy; Genentech: Consultancy. Dalmasso:I-See s.r.l.: Employment. Pierce:Precision Sensing LLC: Equity Ownership. Vitolo:Janssen: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Abbvie: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Novartis: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Celgene: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Juno Therapeutics: Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche: Speakers Bureau; Gilead: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Kite: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau. Martelli:Servier: Honoraria; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffman-La Roche, Celgene, Janssen, Sandoz, Novartis, Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Honoraria. Sehn:Janssen-Ortho: Consultancy, Honoraria; Janssen-Ortho: Honoraria. Trněný:Takeda: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; F. Hoffmann-La Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Celgene: Consultancy; Bristol-Myers Squibb: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Abbvie: Consultancy, Honoraria. Nielsen:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Bolen:Genentech, Inc.: Employment; F. Hoffmann-La Roche: Equity Ownership. Sahin:F. Hoffmann-La Roche Ltd: Employment, Equity Ownership. Lee:Genentech: Employment; F. Hoffman-La Roche: Equity Ownership. El-Galaly:Roche: Employment, Other: Travel support; Takeda: Other: Travel support. Mattiello:F. Hoffmann-La Roche Ltd: Employment. Kinahan:Co-founded PET/X LLC: Equity Ownership; Philips Medical: Research Funding; GE Healthcare: Research Funding; F. Hoffmann-La Roche: Consultancy. Chauvie:International Agency on Atomic Energy (IAEA): Consultancy; Co-owner of Dixit srl (spin-off University of Torino): Equity Ownership; F. Hoffmann-La Roche: Research Funding; Fondazione Cassa di Risparmio di Cuneo (CRC): Research Funding; Italian Foundation on Lymphoma (FIL): Research Funding; Italian Association for Cancer Research (AIRC): Research Funding; SIRTEX: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal