Background: We previously reported that pretreatment with rabbit anti-thymocyte globulin (ATG) decreases the use of immunosuppressive therapy (IST) and occurrence of chronic graft-versus-host disease (GVHD) 12 months after allogeneic stem cell transplantation from unrelated donors. We hypothesized these benefits would persist beyond 12 months with a positive clinical impact on patients.
Methods: Phase 3, multicentre, open-label, randomized controlled trial at 10 centres in Canada and one in Australia. Patients aged 16-70 years with a hematological malignancy, a matched (HLA-A, B, C and DRB1) or 1-antigen/allele mismatched unrelated donor were eligible. Myeloablative, nonmyeloablative or reduced intensity conditioning regimens were permitted according to center clinical preference. Patients were randomized to receive or not to receive rabbit ATG (Thymoglobulin®, Sanofi Canada) as part of their conditioning. GVHD prophylaxis included either cyclosporine or tacrolimus plus methotrexate or mycophenolate mofetil. The ATG arm received 0.5, 2.0, 2.0 mg/kg of ATG on days -2, -1 and +1, respectively. Analyses were on a modified intention-to-treat basis for patients actually transplanted. Primary endpoint was freedom from all systemic IST without resumption up to 24 months after transplantation. Secondary endpoints included survival, relapse, non-relapse mortality, incidence and symptoms of chronic GVHD according to Lee scale and quality of life using different questionnaires including the Center for Epidemiologic Studies Depression (CES-D) scale. We also aimed to evaluate the recently described endpoints of graft-versus-host disease and relapse-free survival (GRFS) and chronic graft-versus-host disease and relapse-free survival (CRFS) in each cohort. This trial was registered at ISRCTN (#29899028) and clinicaltrials.gov (#NCT01217723).
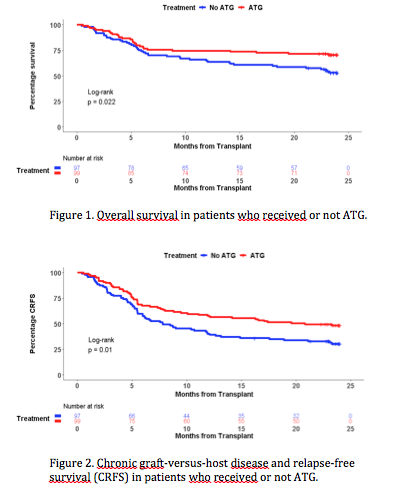
Results: Between 06/2010 and 07/2013, 203 patients were randomized and 196 available for end-points analysis, including 99 patients in the ATG group and 97 in the No ATG (control) group. Datalock was performed on April 1, 2019. The cumulative incidence of chronic GVHD at 24 months was significantly lower in ATG recipients (26.3% versus 41.2%, p=0.032). Similarly, more than twice patients in the ATG group were free from IST at 24 months (adjusted OR of 3.49 [95% CI : 1.60-7.60]; p = 0.002). Most patients retained the same IST status from 12 to 24 months (74.7% in the ATG and 81.4% in the control group). Symptoms of chronic GVHD were also significantly less prevalent in patients receiving ATG, with scores by Lee scale of 13.57 (SE : 1.47) versus 19.90 (SE : 2.15); p=0.017. In contrast, we observed no difference in non-relapse mortality (ATG : 21.2% versus No ATG : 30.9%; p=0.14) and relapse (ATG : 16.2% versus No ATG : 17.5%; p=0.73). Of note, there was no increase in relapse in those receiving either myeloablative or non-myeloablative conditioning (Gray's test p = 0.66 and 0.29, respectively). ATG had a positive impact on survival (Figure 1), with an overall survival at 12 months of 74.8% (SE : 4.4) compared with 64.9% (SE : 4.8) in the control group (adjusted HR 0.56 [95% CI : 0.35-0.90; p=0.017). This benefit of ATG on survival persisted at 24 months, with 70.7% of patients in the ATG group and 53.6% in the control group being alive (p=0.018). GRFS at 12 and 24 months were significantly better in the ATG group, with 45.4% and 37.4% of patients alive and free of ever having had GVHD versus 24.7% and 17.5%, respectively (p = 0.0034). CRFS led to similarly better results in ATG recipients at 12 (57.6%) and 24 (48.5%) months (p=0.01; Figure 2). Depressive symptoms were less frequently reported in the ATG group, the mean CES-D scores being 10.39 (SE : 1.29) compared with 14.63 (SE : 1.48) in the No ATG group (p=0.034). There were no statistically significant differences in other patient-reported outcomes.
Conclusions: Pretreatment with rabbit ATG in combination with standard acute GVHD prophylaxis provides long term benefits consisting of decreases in chronic GVHD incidence, use of IST, depression and improved survival. Our trial is the first to demonstrate both a survival advantage and improvement in quality of life in patients receiving ATG for chronic GVHD prophylaxis. Our data support that ATG should be included in the preparative regimens of all unrelated donor transplant recipients receiving standard acute GVHD prophylaxis.
Roy:Celgene: Consultancy, Honoraria, Research Funding; ExCellThera: Patents & Royalties: Royalties from sales of UM171, Research Funding; Amgen Canada: Honoraria; Janssen Canada: Honoraria; Sanofi Canada: Research Funding. Foley:Celgene: Speakers Bureau; Janssen: Speakers Bureau; Amgen: Speakers Bureau. Kuruvilla:Janssen: Research Funding; Roche: Research Funding; BMS: Consultancy; Abbvie: Consultancy; Gilead: Consultancy; Karyopharm: Consultancy; Merck: Consultancy; Roche: Consultancy; Seattle Genetics: Consultancy; Amgen: Honoraria; Astra Zeneca: Honoraria; BMS: Honoraria; Celgene: Honoraria; Gilead: Honoraria; Janssen: Honoraria; Karyopharm: Honoraria; Merck: Honoraria; Novartis: Honoraria; Roche: Honoraria; Seattle Genetics: Honoraria. Lee:AstraZeneca: Research Funding; Incyte: Research Funding; Syndax: Research Funding; Amgen: Research Funding; Novartis: Research Funding; Takeda: Research Funding; Kadmon: Consultancy, Research Funding; Pfizer: Consultancy, Research Funding. Popradi:Sanofi Canada: Consultancy, Honoraria. Walker:Kiadis Pharma: Other: Grant funding via institution (as a principal investigator).
Rabbit ATG (Sanofi) for chronic GVHD prophylaxis.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal