Background: Immune thrombocytopenia (ITP) is characterized by immune-mediated platelet destruction and impairment of platelet production, leading to downstream thrombocytopenia, a predisposition to bleeding, and adverse impact on patient quality of life. Unmet needs in relapsed or refractory ITP are to improve remission rates and durability through targeting underlying disease mechanisms. PRN1008 is an oral, reversible, covalent inhibitor of Bruton tyrosine kinase (BTK) that modulates immune-mediated processes in ITP. Preclinical PRN1008 data showed inhibition of B-cell receptor-mediated activation of human B cells, Fc receptor (Fc-gamma and Fc-epsilon)-mediated activation of immune cells, and dose-dependent reduction in platelet loss in a mouse ITP model. In platelets from normal healthy volunteer and ITP patients, clinically-relevant concentrations of PRN1008 showed no platelet aggregation or interference with other platelet agonists, in contrast to ibrutinib (Langrish et al. ASH 2017:1052).
Methods: This is an ongoing open-label, adaptive, intra-patient dose-escalation, phase I/II study of PRN1008 in adult patients with relapsed or refractory ITP (primary or secondary) who previously responded to ≥ 1 prior ITP therapy and have no available therapeutic options (NCT03395210). Eligible patients have two platelet counts < 30,000/µL within 15 days prior to treatment. Oral PRN1008 starting doses were 200 mg QD, 400 mg QD, 300 mg BID (total 600 mg daily), and 400 mg BID (total 800 mg daily), with intra-patient dose escalation allowed every 4 weeks (maximum 400 mg BID) as needed for efficacy. Stable doses of concomitant corticosteroids and thrombopoietin-receptor agonists (TPO-RA) are permitted. The primary end point is the proportion of patients with ≥ 2 consecutive platelet counts (separated by ≥ 5 days) of ≥ 50,000/µL and increased by ≥ 20,000/µL from baseline without requiring rescue medication.
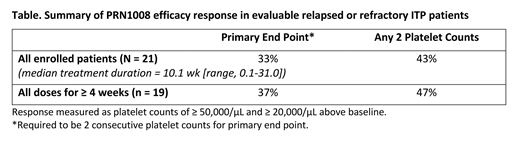
Results: A total of 21 patients have been enrolled to date at starting doses of 200 mg QD (n=9), 400 mg QD (n=1), 300 mg BID (n=5), and 400 mg BID (n=6). As of 15 July 2019 data cut-off, 11 patients were receiving ongoing treatment, 4 completed the study, and 6 patients withdrew (2 due to patient decision, 2 from non-treatment-related adverse events [AEs], 1 erroneously enrolled, and 1 because of rescue medication use). Patients had a median age of 54 y (range, 30-65), 4 (19%) had a prior splenectomy, 19 (90%) were diagnosed with primary ITP, and 2 (10%) with secondary ITP. Patients had ITP for a median of 8.3 years (range, 0.5-42.4) and had received a median of 4 prior ITP therapies. Median platelet count at study entry was 14,173/µL (range, 2,670-27,000/µL). During the study, 6 (29%) patients received PRN1008 monotherapy; 15 (71%) patients were on ≥ 1 concomitant ITP medication. Related treatment-emergent AEs (TEAEs) were reported by 4 (19%) patients; all were grade 1 or 2. The most frequent related TEAEs were nausea, diarrhea, and abdominal distension. There were no treatment-related bleeding or thrombotic events, and no significant changes in the ITP-BAT bleeding scale between baseline and the last visit. There were no dose limiting toxicities (DLT). Patients had received treatment for a median of 10.1 weeks (range, 0.1-31.0). Overall, 7 (33%) patients achieved the primary endpoint across all doses (Table). Patient responses were improved at the 2 higher doses. In 10 patients who had reached ≥ 12 weeks of treatment, ≥ 50% of patients had platelet counts of ≥ 50,000/µL and ≥ 20,000/µL increases from baseline.
Conclusion: Overall, PRN1008 was active in 33% of ITP patients who were refractory to multiple treatments with no alternative therapeutic options. This result was demonstrated despite the limited duration of treatment and including patients at all dose levels. In addition, patients treated for longer periods of time have substantially improved response rates that support continued interest in this ongoing study. The safety profile was tolerable at all studied doses whether given as a monotherapy or with allowed concomitant ITP therapy. Importantly, TEAEs were grade 1 or 2 with no thrombotic events. The dose-escalation portion of the study is complete; enrollment is expanding at the 400 mg BID starting dose for a duration of 24 weeks to further characterize treatment benefit and for continued treatment beyond 24 weeks in patients who have responded.
Kuter:Dova: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Caremark: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Kezar: Research Funding; Argenx: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria; Platelet Disorder Support Association: Consultancy, Honoraria; Principia: Consultancy, Honoraria, Research Funding; Alnylam: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Shinogi: Consultancy, Honoraria; Shire: Consultancy, Honoraria; Merck Sharp Dohme: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Protalix: Consultancy, Honoraria; Rigel: Consultancy, Honoraria, Research Funding; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; UCB: Consultancy, Honoraria; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Zafgen: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding. Boccia:AstraZeneca: Speakers Bureau; Celgene: Speakers Bureau; Amgen: Speakers Bureau; AMAG: Consultancy; Genentech: Speakers Bureau; DSI: Speakers Bureau. Lee:Weill Cornell Medical College: Employment. Tzvetkov:UMHAT Georgi Stranski: Employment; DCC Pleven: Consultancy. Mayer:AOP Orphan Pharmaceuticals AG: Research Funding. Trněný:Abbvie: Consultancy, Honoraria; Gilead Sciences: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; Bristol-Myers Squibb: Consultancy, Honoraria; MorphoSys: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy; F. Hoffmann-La Roche: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Kostal:Novartis: Honoraria; AOP: Honoraria; University Hospital in Hradec Kralove, Czech Republic: Employment. Hajek:Janssen: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; AbbVie: Consultancy; BMS: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Research Funding; PharmaMar: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding, Speakers Bureau. McDonald:Bayer: Honoraria; Amgen: Honoraria; Novartis: Honoraria. Bandman:Principia Biopharma: Employment, Equity Ownership, Patents & Royalties: Institutional with Incyte and Portola, no royalties. Burns:Principia BioPharma: Employment. Neale:Principia BioPharma: Employment, Equity Ownership. Thomas:Principia Biopharma: Employment, Equity Ownership; BMS: Equity Ownership; Pfizer: Equity Ownership. Cooper:Principia: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Rigel: Consultancy, Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees.
Yes, this was an investigational clinical phase I/II study of PRN1008 in patients with relapsed/refractory ITP. Phase I dose escalation phase is now complete and expanded phase II studies ongoing.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal