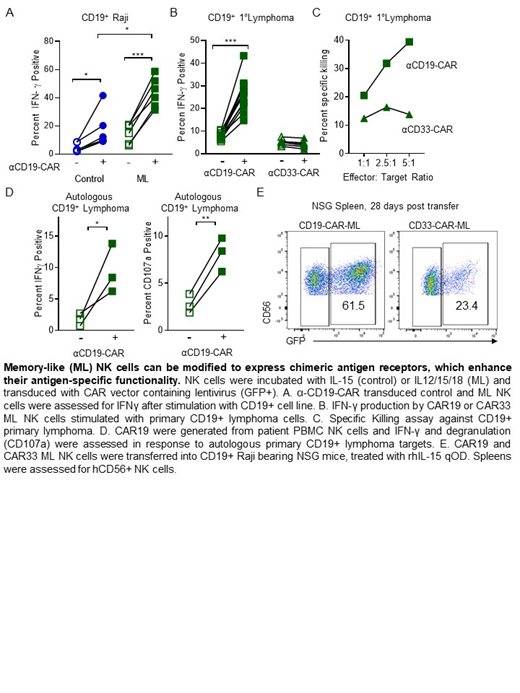
NK cells are cytotoxic innate lymphoid cells that mediate anti-tumor responses and traffic to most tissues. NK cells activated briefly with IL-12, IL-15, and IL-18 differentiate into memory-like (ML) NK cells with enhanced anti-tumor effects, which has been translated into clinical trials for patients with leukemia. Adoptive cellular therapy with donor ML NK cells were safe and induced CR/CRi in >50% of rel/ref AML patients in a first-in-human phase 1 clinical trial at Washington University (PMID27655849). In preliminary results, donor ML NK cells expand and can persist for months in an immune-compatible recipients during or after allogeneic hematopoietic cell transplantation (HCT), and maintain potent effector function. However, NK cell recognition of many cancer types is limited, since they depend on expression of stress-induced activating receptor ligands. We hypothesized that ML NK cells engineered with chimeric antigen receptors (CAR) would demonstrate improved anti-tumor responses against classically NK-resistant targets. To test this idea, ML NK cells were engineered to express an anti-CD19-CAR, and responses against NK-resistant B-cell malignancies evaluated in vitro and in vivo. CAR-modified primary human ML NK cells (CAR-ML) were transduced with an anti-CD19-CD8a-41BB-CD3z-GFP (CD19-CAR-ML) lentivirus. This differentiation and transfection approach resulted in approximately 15-25% of ML NK cells transduced with the CAR construct, as determined flow cytometry staining for soluble CD19 and GFP. As an additional control, αCD33-CD8a-41BB-CD3z-GFP CAR-transduced ML NK cells and GFP- internal control ML NK cells were used.
Here, in vitro functional assays were used to determine if CD19-CAR enhances ML NK cells in an antigen (CD19)-specific manner. CD19-CAR-ML (GFP+) and control ML NK (GFP-) cells were evaluated for functional responses to CD19-positive or CD19-negative tumor targets in vitro. CD19-CAR-ML NK cells demonstrated significantly increased IFN-γ production (44±4% vs. 15±3%, p<0.001); mean ± SEM) and degranulation (31±4% vs. 5±1%, p<0.001) against NK-resistant CD19+ Raji targets, compared to control GFP- and CD33-CAR ML NK cells. To understand the contributions of ML differentiation on the enhanced functionality of CD19-CAR modified NK cells, we compared CD19-CAR-ML NK cells to control CD19-CAR NK cells that were treated with IL-15 only. CD19-CAR-ML NK cells also exhibited significantly increased effector responses compared to control CAR NK cells against CD19+ targets (p<0.01). CD19-CAR-ML NK cells responded similarly to GFP- ML NK cells against CD19-negative Kasumi leukemia targets. Finally, CD19-CAR-ML NK cells also exhibited significantly enhanced killing, degranulation, and IFN-γ production against primary CD19+ follicular lymphoma targets from patient lymph nodes (p<0.01). To establish the translational utility of this approach, autologous CD19-CAR-ML NK cells generated from lymphoma patients demonstrated significantly increased IFN-γ production (p<0.05) and degranulation (p<0.01) against their own CD19+ lymphoma targets, compared to control ML NK cells (GFP-). These data confirm contributions of both ML differentiation and CAR expression in the enhanced antigen-specific, anti-tumor responses observed in CAR-ML NK cells.
To test the expansion and persistence of ML NK cells transduced with CD19 or CD33-CAR were transferred into CD19+ Raji-bearing NSG mice and supported in vivo with IL-15. After three weeks, mice were sacrificed and NK (CD56) cell persistence and tumor (CD19) burden assessed by flow cytometry. CD19-CAR ML recipient mice had reduced tumor burden in the BM, spleen, and blood compared to CD33-CAR ML treated mice. Notably, CD19-CAR-ML (GFP+) were increased to >70% of human NK cells from 20% in the CD33-CAR-ML recipient mice, suggesting antigen-specific CAR-expressing NK cells expand or survive better in vivo than non-transduced ML or non-specific CAR-ML NK cells in vivo. Thus, combining CAR with ML differentiation results in NK cells with enhanced responses to NK resistant tumors. These studies warrant continued CAR-ML development and provide the pre-clinical rationale for translating this combination NK cell therapy approach to the clinic.
Fehniger:Cyto-Sen Therapeutics: Consultancy; Horizon Pharma PLC: Other: Consultancy (Spouse).
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal