Background: DARA, a human IgGκ monoclonal antibody targeting CD38, is approved in combination with bortezomib, melphalan, and prednisone (VMP) and bortezomib and dexamethasone (Vd) for newly diagnosed MM (NDMM) and relapsed MM (RMM), respectively. CyBorD is a commonly used immunomodulatory drug-sparing regimen for MM. In the LYRA (NCT02951819) study, DARA plus CyBorD (DARA-CyBorD) demonstrated efficacy and a tolerable safety profile at the end of induction. Here, we present updated findings examining the effect of monthly DARA maintenance on the efficacy and safety of DARA-CyBorD in NDMM and RMM.
Methods: LYRA is an ongoing, single-arm, open-label, phase 2 study conducted at US community oncology centers. Patients (pts) were aged ≥18 years with documented MM per IMWG criteria, an ECOG performance score (PS) of 0-2, and ≤1 prior line of therapy. Pts received 4-8 induction cycles of DARA-CyBorD (cyclophosphamide 300 mg/m2 PO on Days 1, 8, 15, and 22; bortezomib 1.5 mg/m2 SC on Days 1, 8, and 15; and dexamethasone 40 mg PO or IV weekly [qw]) every 28 days. DARA was given at 8 mg/kg IV on Days 1 and 2 of C1, 16 mg/kg qw from C1D8 through C2, 16 mg/kg q2w for C3-6, and 16 mg/kg q4w for C7-8. After induction, eligible pts could undergo autologous stem cell transplantation (ASCT). All pts received up to 12 maintenance cycles with DARA 16 mg/kg IV q4w.
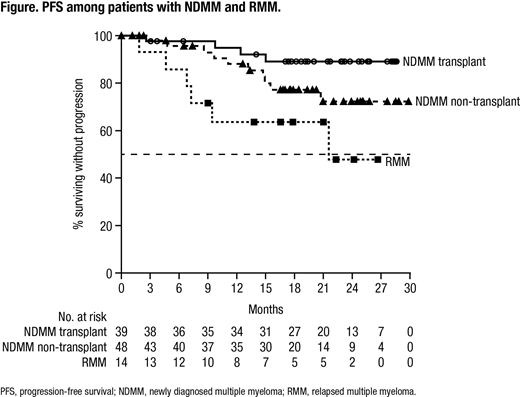
Results: A total of 101 (87 NDMM, 14 RMM) pts were enrolled; 100 (86 NDMM, 14 RMM) pts received ≥1 treatment dose. Median age was 63 years; most pts were white (81%), male (64%), had ECOG PS 0-1 (94%) and had IgG (57%) MM; 36% of pts had high cytogenetic risk, defined as a del(17p), t(4:14) or t(14;16) abnormality. NDMM and RMM pts received a median of 6 and 8 cycles, respectively, of induction therapy. Thirty-nine NDMM pts and 1 RMM pt underwent ASCT. Fifty percent of pts received plerixafor; median stem cell yield for NDMM pts was 6.2 x 106 (range 2-15 x 106) CD34+ cells/kg. A total of 85 (75 NDMM, 10 RMM) pts received ≥1 dose of maintenance treatment; 63 (56 NDMM, 7 RMM) pts have received all 12 maintenance cycles. In NDMM pts, ORR was 87%, with 64% ≥VGPR and 12% ≥CR, by the end of induction. By the end of maintenance, ORR, ≥VGPR and ≥CR rates were 97%, 82% and 51% in NDMM pts who underwent ASCT and 83%, 70% and 30% in NDMM pts who did not receive ASCT. In RMM pts, ORR, ≥VGPR and ≥CR rates were 79%, 71% and 29% by the end of induction and 86%, 71% and 64% by the end of maintenance. At a median follow up of 24.8 mo in NDMM pts and 26.6 mo in RMM pts, median duration of response was not reached (NR). Median PFS (Figure) was NR in NDMM pts, regardless of transplant status, and was 21.7 mo in RMM pts; median OS was NR in NDMM pts and was 30.1 mo in RMM pts. In NDMM pts the 24-mo PFS rate was 89% in pts who underwent ASCT and 72% in pts who did not receive ASCT. The 24-mo OS rate was 90% for NDMM pts. In RMM pts, the 24-mo PFS and OS rates were 48% and 64%, respectively. All treated pts had ≥1 TEAE. Common TEAEs (≥25%) included fatigue, nausea, cough, diarrhea, upper respiratory tract infection, back pain, vomiting, insomnia, dyspnea, constipation, and headache. Grade 3/4 TEAEs were reported in 62% of pts; the most common (≥10%) was neutropenia (14%). Serious TEAEs occurred in 33% of pts; the most common (>2%) were pneumonia, atrial fibrillation and pulmonary embolism. TEAEs led to permanent treatment discontinuation in 7% of pts, with 2% related to treatment. TEAEs resulted in death in 2 pts (nephrotic syndrome, sudden death); both unrelated to treatment. Infusion reactions (IRs) occurred in 56% of pts including grades 1-2 in 52% of pts, grade 3 in 3% of pts and grade 4 in 1% of pts. Most common (>5%) IRs were chills, cough, dyspnea, nausea, pruritus, flushing and nasal congestion.
Conclusion: Maintenance with DARA monotherapy for 12 mo increased the >CR rate in NDMM and RMM pts, consistent with observations in prior studies that longer DARA treatment improves depth of response. Importantly, the increase in ≥CR rate was associated with durable PFS and OS. The 24-mo PFS rates in NDMM and RMM pts compare favorably with results for DARA-VMP and DARA-Vd in NDMM and RRMM, respectively. Safety profile was consistent with previous reports of DARA, with no new safety concerns observed with longer follow-up. These data indicate that DARA-CyBorD is a safe, effective MM treatment and that DARA maintenance increases depth of response and achieves durable remissions.
Rifkin:Amgen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Takeda: Membership on an entity's Board of Directors or advisory committees. Melear:Texas Oncology: Employment; DARA: Speakers Bureau. Faber:Cardinal Health: Consultancy, Honoraria; Celgene: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Kite: Consultancy, Honoraria; Amgen: Consultancy, Honoraria. Bensinger:Amgen, Celgene: Other: Personal Fees, Research Funding, Speakers Bureau; Takeda, Janssen: Speakers Bureau; Sanofi, Seattle Genetics, Merck, Karyopharm: Other: Grant. Burke:Gilead: Consultancy; Celgene: Consultancy; Roche/Genentech: Consultancy. Narang:Celgene: Speakers Bureau. Stevens:Astellas: Consultancy. Gunawardena:Janssen: Employment, Equity Ownership. Lutska:Janssen: Employment. Qi:Janssen: Employment. Ukropec:Janssen: Employment, Equity Ownership. Qi:Janssen: Employment. Lin:Janssen: Employment, Equity Ownership. Yimer:Amgen: Consultancy; Clovis Oncology: Equity Ownership; Puma Biotechnology: Equity Ownership; Celgene: Honoraria; Seattle Genetics: Honoraria; Janssen: Speakers Bureau; AstraZeneca: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal