
Background Chronic myelomonocytic leukemia (CMML) is an ultrarare stem cell disorder defined by the presence of monocytosis (≥1.0 G/l, ≥10%). Depending on white blood cell (WBC) count, CMML can be divided into a myelodysplastic (MD) (WBC ≤13 G/l) and a myeloproliferative (MP) variant (WBC >13 G/l). Although hypomethylating agents (HMA) have been shown to prolong overall survival (OS) in MDS patients (pts) in prospective, randomized phase III trials, only 6-14 MD-CMML pts were included, and MP-CMML pts were excluded [Silverman 2002; Kantarjian 2006; Fenaux 2009]. EMA approval of azacitidine (AZA) in CMML is thus based on limited experience and restricted to MD-CMML with 10-29% bone marrow blasts (BMB), whereas decitabine (DAC) is not approved for treatment (trt) of CMML in the EU. Smaller analyses and single-arm trials of HMA in CMML exist [Wijermans 2008; Ades 2013; Pleyer 2014; Zeidan 2017; Duchmann 2018; Santini 2018; Coston 2019; Diamantopoulos 2019], but it is still unclear whether HMA provide a benefit in CMML (subgroups) compared with other trts.
Aim Evaluate the impact of HMA and hydroxyurea (HU) trt on OS and time to next trt (TTNT).
Methods Data were collected from 7 European study groups and 2 US MDS Centers of Excellence; database lock 27.05.19; Assign Data Management and Biostatistics GmbH performed statistical analyses with SAS® 9.3.
Of 1657 CMML pts, only those who received trt (n=950), with documented WBC and BMB at 1st line, were included in these analyses (n=845, cohort 1). Pts were stratified according to the EMA approved AZA indication, and inclusion/exclusion criteria of the GFM-DAC-CMML trial assessing DAC +/- HU vs HU (NCT02214407) (diagnosis of CMML, no prior trt [except supportive care, erythropoietin or ≤6 weeks HU], WBC ≥13 G/l and ≥2 of the following: BMB ≥5%, clonal cytogenetic abnormality [other than -Y], hemoglobin <10 g/dL, neutrophil count >16 G/l, platelet count <100 G/L, splenomegaly; pts with ECOG>2 excluded) (n=486; cohort 2).
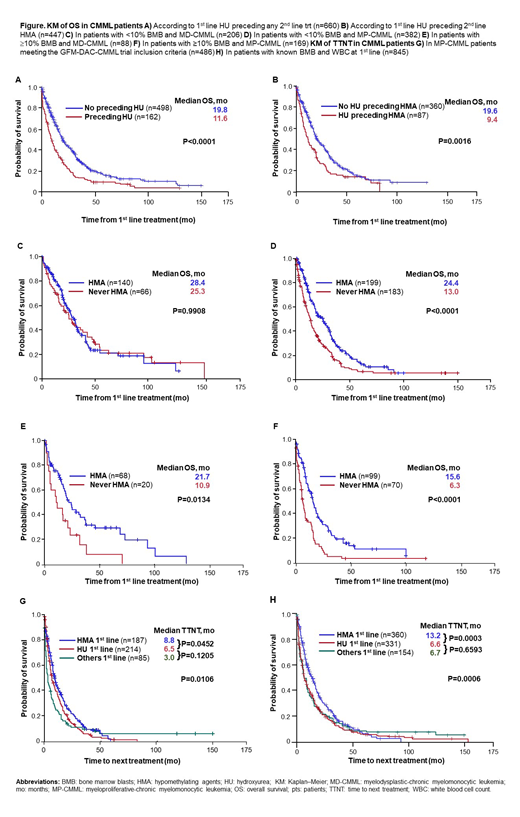
Results In cohort 1, pts receiving HMA 1st line (n=375) had longer OS (19.8 vs 16.3 months [mo], P=0.0102) and TTNT (13.2 vs 6.7 mo, P=0.0001) than pts treated with non-HMA 1st line (n=470). Survival benefit was longer when comparing pts who received HMA (any time) (AZA [n=442], DAC [n=37], both [n=27]) with those that never received HMA (never HMA; n=339) (23.0 vs 13.0 mo, P<0.0001). Median OS was longer for MD-CMML (n=294) vs MP-CMML pts (n=551) (25.5 vs 15.0 mo, P<0.0001). OS was shorter for all pts with 1st line HU preceding any 2nd line trt (9.4 vs 19.6 mo; P<0.0001; Fig A), for MP-CMML pts separately (8.7 vs 15.6 mo, P=0.0001), and for the subset with HU preceding 2nd line HMA (11.6 vs 19.8 mo; P=0.0016; Fig B).
The following were significantly less common in pts treated with HMA vs those that were not: diagnosis in the pre-HMA era (8 vs 43%), MP-CMML (48 vs 66%), splenomegaly (27 vs 36%), ECOG≥2 (12 vs 24%), 1 trt line (43 vs 74%). WHO subtype, karyotype, transfusion dependence, LDH, CPSS score, AML transformation and therapy-related CMML were comparable between cohorts.
HMA are not approved in the EU for CMML pts with <10% BMB. In this subgroup (n=588), median OS was longer for MD-CMML vs MP-CMML (28.1 vs 17.0 mo, P<0.0001) and for pts who received HMA vs never HMA (26.5 vs 14.8 mo, P=0.0003). Pts with <10% BMB and MD-CMML (n=206) did not seem to benefit from HMA vs non-HMA trt (median OS 28.4 vs 25.3 mo, P=0.9908; Fig C), whereas the MP-CMML subgroup (n=382) did (24.4 vs 13.0 mo, P<0.0001; Fig D).
HMA are also unapproved in the EU for MP-CMML pts with ≥10% BMB. In pts with ≥10% BMB (n=257), median OS was longer for MD-CMML vs MP-CMML (19.4 vs 11.2 mo, P=0.0023) and for pts who received HMA vs never HMA (18.3 vs 7.0 mo, P<0.0001). Both MD-CMML (OS 21.7 vs 10.9 mo, p=0.0134; Fig E) and MP-CMML pts (15.6 vs 6.3 mo, P<0.0001; Fig F) benefited from HMA trt vs never HMA.
In cohort 2, 1st line trts were HU (n=214), HMA (n=187) and others (n=85). Comparing HMA vs HU 1st line, median OS was 15.6 vs 14.5 mo (P=0.0307) and median TTNT was 8.8 vs 6.5 mo (P=0.0452; Fig G). OS and TTNT were comparable for HU vs other trts (Fig G). Similar observations were made in the larger cohort 1 (Fig H).
Conclusions HMA show promising results with survival benefits of +11.4, +10.8 and +9.3 mo in pts with MP-CMML <10%, and MD- or MP-CMML ≥10% BMB. In MP-CMML pts fulfilling GFM-DAC-CMML trial inclusion criteria, survival and TTNT were longest in pts receiving HMA 1st line as compared to HU or other trts. Preceding HU portends poor prognosis (-10.2 mo).
Pleyer:Abbvie: Other: Advisory board; Novartis: Other: Advisory board; Inflection Point Biomedical Advisors: Other: Advisory board; Celgene: Other: Advisory board; Agios: Other: Advisory board. Leisch:Novartis: Honoraria, Other: Travel support; Bristol-Myers-Squibb: Honoraria; Celgene: Other: Travel support. Maciejewski:Alexion: Consultancy; Novartis: Consultancy. Kaivers:Jazz Pharmaceuticals: Other: Travel Support. Heibl:Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Honoraria; Roche: Honoraria; Daiichi Sankyo: Honoraria; Mundipharma: Honoraria; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; AOP Orphan Pharmaceuticals: Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees. Geissler:Novartis: Honoraria; Roche: Honoraria; Abbvie: Honoraria; AstraZeneca: Honoraria; AOP: Honoraria; Celgene: Honoraria; Pfizer: Honoraria; Amgen: Honoraria; Ratiopharm: Honoraria. Valent:Blueprint: Research Funding; Pfizer: Honoraria; Deciphera: Honoraria, Research Funding; Celgene: Honoraria; Novartis: Consultancy, Honoraria, Research Funding. Medina de Almeida:Novartis: Speakers Bureau; Celgene: Speakers Bureau. Jerez:Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria. Germing:Novartis: Honoraria, Research Funding; Amgen: Honoraria; Celgene: Honoraria, Research Funding; Jazz Pharmaceuticals: Honoraria. Sekeres:Celgene: Membership on an entity's Board of Directors or advisory committees; Millenium: Membership on an entity's Board of Directors or advisory committees; Syros: Membership on an entity's Board of Directors or advisory committees. List:Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding. Symeonidis:Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Tekeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Sanz:AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Amgen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Boehringer-Ingelheim: Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Helsinn Healthcare: Membership on an entity's Board of Directors or advisory committees, Research Funding; Hoffman - La Roche: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen - Cilag: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Onconova: Membership on an entity's Board of Directors or advisory committees, Research Funding. Greil:Boehringer Ingelheim: Honoraria; Amgen: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; AstraZeneca: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding; Janssen-Cilag: Honoraria; Mundipharma: Honoraria, Research Funding; Merck: Consultancy, Honoraria, Research Funding; Eisai: Honoraria; Genentech: Honoraria, Research Funding; Novartis: Consultancy, Honoraria, Other: Travel/accomodation expenses, Research Funding.
Azacitidine is not approved for the treatment of MP-CMML or CMML with <10% BM blasts, decitabine is not approved for treatment of CMML in the EU, hydroxyurea is not approved for the treatment of CMML in the EU.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal